

What makes Pearson 10M So Fast And Strong
Pearson 10M for Sale.
Year: 1979 Price: -
Make: Pearson Model: 10M
LOA: 33.04 Beam: 11
LWL: 29.17 Draft: 5.92
Ballast: 5445 Lbs Displacement: 12441 Lbs
Rig and Sails:
This boat is a Sloop with a Mast-head Rig, and 524 sq. feet of sail area. She has 3 Sails, Including Main Sail, Genoa, Spinnaker.
She has a Fin-keel. The Hull is Original Gel-coat, and is in Recently Restored condition.
1 Yanmar 3GM30F Diesel Engine, 24 horse power with 500 Hours.
Nav-Station w/ Garmin GPS (2014), New Stereo System,VHF Radio (2014), Depth Sounder and Speed,Tack-tick MN30 Indicator and windex, Hood Genoa Furling System, Harken Main-sheet Traveller, Richie Compass, new cushion covers (2014), 6 Winches, Stainless-steel Swim-ladder, New Stainless-steel Shaft (2017), New 3-bladed Prop (2017), folding racing prop, Yanmar 3GM30F Diesel (2000), Spinnaker Pole and track w/ ring, Boat Hook, Fenders (3), Life-jackets (3), Flare Kit, Air Horn, First Aid Kit.
This is the Yacht that will take you away to seemingly unreachable destinations, that until now existed only between the covers of your favorite cruising magazine. That's because this Pearson 10M has the muscle to sail through harsh conditions, and the speed to make it in timely fashion. That means you'll spend less time tending to her, and more time enjoying the sites and sounds of places you could only dream of. Her tall rig and long waterline enhanced by a set of sails for racing and a set for cruising, a modern array of navigation equipment, and a powerful Yanmar 3GM30F Diesel engine, all come together to make this vessel a one of a kind cruising machine. On deck she is solid, with a large cockpit, and easy to control wheel helm. You will have an easier time piloting, because with all running rigging lead aft to the cockpit, there's no need to leave the comforts of her Bimini covered helm station. That means a sun-shade for protection, updated stereo sound system, pedestal mounted GPS and cup holders, all within reach of your comfy wheel-helm. This is your time to make the most of the sailing season. So don't miss your chance to buy this magnificent yacht, the Pearson 10M.
Contact Fred for arrangements and info: 3479273350
Or email for more details: [email protected]
Photo Gallery: Pearson 10M
| boatTitleImage |
| Exterior Profile |
| Exterior Forward |
| Exterior Aft |
| Cockpit |
| Keel |
| Companionway |
| Cabin Looking Aft |
| Cabin Starboard |
| Cabin Port |
| V-Birth |
| Shower/ Head |
| Head |
This listing Originated at Barronsmarine.com/boats-for-sale/ .
For questions on listings Call: (347) 927-3350 Email: [email protected]

Review of Pearson 10M
Basic specs..
The hull is made of fibreglass. Generally, a hull made of fibreglass requires only a minimum of maintenance during the sailing season.
The boat is equipped with 150.0 liter fresh water capacity.
The boat equipped with a masthead rig. The advantage of a masthead rig is its simplicity and the fact that a given sail area - compared with a fractional rig - can be carried lower and thus with less heeling moment.
The Pearson 10M is equipped with a fin keel. A boat with a fin keel is more manoeuvrable but has less directional stability than a similar boat with a long keel.
The boat can only enter major marinas as the draft is about 1.80 - 1.90 meter (5.91 - 6.21 ft) dependent on the load. See immersion rate below.
Pearson 10M is typically equipped with a Universal Atomic 4 gasoline engine at 30.0 hp (22 kW), which gives a max speed about 6.2 knots.
The fuel tank has a capacity of 75.0 liters (19 US gallons, 16 imperial gallons).
Sailing characteristics
This section covers widely used rules of thumb to describe the sailing characteristics. Please note that even though the calculations are correct, the interpretation of the results might not be valid for extreme boats.
What is Capsize Screening Formula (CSF)?
The capsize screening value for Pearson 10M is 1.90, indicating that this boat could - if evaluated by this formula alone - be accepted to participate in ocean races.
What is Theoretical Maximum Hull Speed?
The theoretical maximal speed of a displacement boat of this length is 7.2 knots. The term "Theoretical Maximum Hull Speed" is widely used even though a boat can sail faster. The term shall be interpreted as above the theoretical speed a great additional power is necessary for a small gain in speed.
The immersion rate is defined as the weight required to sink the boat a certain level. The immersion rate for Pearson 10M is about 199 kg/cm, alternatively 1114 lbs/inch. Meaning: if you load 199 kg cargo on the boat then it will sink 1 cm. Alternatively, if you load 1114 lbs cargo on the boat it will sink 1 inch.
Sailing statistics
This section is statistical comparison with similar boats of the same category. The basis of the following statistical computations is our unique database with more than 26,000 different boat types and 350,000 data points.
What is Motion Comfort Ratio (MCR)?
What is L/B (Length Beam Ratio)?
What is a Ballast Ratio?
What is Displacement Length Ratio?
What is SA/D (Sail Area Displacement ratio)?
Maintenance
When buying anti-fouling bottom paint, it's nice to know how much to buy. The surface of the wet bottom is about 34m 2 (365 ft 2 ). Based on this, your favourite maritime shop can tell you the quantity you need.
Are your sails worn out? You might find your next sail here: Sails for Sale
If you need to renew parts of your running rig and is not quite sure of the dimensions, you may find the estimates computed below useful.
| Usage | Length | Diameter | ||
| Mainsail halyard | 30.6 m | (100.5 feet) | 10 mm | (3/8 inch) |
| Jib/genoa halyard | 30.6 m | (100.5 feet) | 10 mm | (3/8 inch) |
| Spinnaker halyard | 30.6 m | (100.5 feet) | 10 mm | (3/8 inch) |
| Jib sheet | 10.1 m | (33.0 feet) | 12 mm | (1/2 inch) |
| Genoa sheet | 10.1 m | (33.0 feet) | 12 mm | (1/2 inch) |
| Mainsheet | 25.2 m | (82.5 feet) | 12 mm | (1/2 inch) |
| Spinnaker sheet | 22.1 m | (72.6 feet) | 12 mm | (1/2 inch) |
| Cunningham | 3.4 m | (11.0 feet) | 10 mm | (3/8 inch) |
| Kickingstrap | 6.7 m | (22.0 feet) | 10 mm | (3/8 inch) |
| Clew-outhaul | 6.7 m | (22.0 feet) | 10 mm | (3/8 inch) |
This section is reserved boat owner's modifications, improvements, etc. Here you might find (or contribute with) inspiration for your boat.
Do you have changes/improvements you would like to share? Upload a photo and describe what you have done.
We are always looking for new photos. If you can contribute with photos for Pearson 10M it would be a great help.
If you have any comments to the review, improvement suggestions, or the like, feel free to contact us . Criticism helps us to improve.
Great choice! Your favorites are temporarily saved for this session. Sign in to save them permanently, access them on any device, and receive relevant alerts.
- Sailboat Guide
Pearson 10M
Pearson 10M is a 33 ′ 0 ″ / 10.1 m monohull sailboat designed by William Shaw and built by Pearson Yachts between 1973 and 1981.
- 2 / 28 Lewes, DE, US 1977 Pearson 10M $13,000 USD View
- 3 / 28 Alameda, CA, US 1975 Pearson 10M $27,900 USD View
- 4 / 28 Fall River, MA, US 1977 Pearson 10M $12,000 USD View
- 5 / 28 Lewes, DE, US 1977 Pearson 10M $13,000 USD View
- 6 / 28 Lewes, DE, US 1977 Pearson 10M $13,000 USD View
- 7 / 28 Alameda, CA, US 1975 Pearson 10M $27,900 USD View
- 8 / 28 Fall River, MA, US 1977 Pearson 10M $12,000 USD View
- 9 / 28 Fall River, MA, US 1977 Pearson 10M $12,000 USD View
- 10 / 28 Lewes, DE, US 1977 Pearson 10M $13,000 USD View
- 11 / 28 Alameda, CA, US 1975 Pearson 10M $27,900 USD View
- 12 / 28 Lewes, DE, US 1977 Pearson 10M $13,000 USD View
- 13 / 28 Alameda, CA, US 1975 Pearson 10M $27,900 USD View
- 14 / 28 Lewes, DE, US 1977 Pearson 10M $13,000 USD View
- 15 / 28 Alameda, CA, US 1975 Pearson 10M $27,900 USD View
- 16 / 28 Lewes, DE, US 1977 Pearson 10M $13,000 USD View
- 17 / 28 Lewes, DE, US 1977 Pearson 10M $13,000 USD View
- 18 / 28 Alameda, CA, US 1975 Pearson 10M $27,900 USD View
- 19 / 28 Alameda, CA, US 1975 Pearson 10M $27,900 USD View
- 20 / 28 Lewes, DE, US 1977 Pearson 10M $13,000 USD View
- 21 / 28 Alameda, CA, US 1975 Pearson 10M $27,900 USD View
- 22 / 28 Lewes, DE, US 1977 Pearson 10M $13,000 USD View
- 23 / 28 Alameda, CA, US 1975 Pearson 10M $27,900 USD View
- 24 / 28 Lewes, DE, US 1977 Pearson 10M $13,000 USD View
- 25 / 28 Alameda, CA, US 1975 Pearson 10M $27,900 USD View
- 26 / 28 Lewes, DE, US 1977 Pearson 10M $13,000 USD View
- 27 / 28 Alameda, CA, US 1975 Pearson 10M $27,900 USD View
- 28 / 28 Alameda, CA, US 1975 Pearson 10M $27,900 USD View
Rig and Sails
Auxilary power, accomodations, calculations.
The theoretical maximum speed that a displacement hull can move efficiently through the water is determined by it's waterline length and displacement. It may be unable to reach this speed if the boat is underpowered or heavily loaded, though it may exceed this speed given enough power. Read more.
Classic hull speed formula:
Hull Speed = 1.34 x √LWL
Max Speed/Length ratio = 8.26 ÷ Displacement/Length ratio .311 Hull Speed = Max Speed/Length ratio x √LWL
Sail Area / Displacement Ratio
A measure of the power of the sails relative to the weight of the boat. The higher the number, the higher the performance, but the harder the boat will be to handle. This ratio is a "non-dimensional" value that facilitates comparisons between boats of different types and sizes. Read more.
SA/D = SA ÷ (D ÷ 64) 2/3
- SA : Sail area in square feet, derived by adding the mainsail area to 100% of the foretriangle area (the lateral area above the deck between the mast and the forestay).
- D : Displacement in pounds.
Ballast / Displacement Ratio
A measure of the stability of a boat's hull that suggests how well a monohull will stand up to its sails. The ballast displacement ratio indicates how much of the weight of a boat is placed for maximum stability against capsizing and is an indicator of stiffness and resistance to capsize.
Ballast / Displacement * 100
Displacement / Length Ratio
A measure of the weight of the boat relative to it's length at the waterline. The higher a boat’s D/L ratio, the more easily it will carry a load and the more comfortable its motion will be. The lower a boat's ratio is, the less power it takes to drive the boat to its nominal hull speed or beyond. Read more.
D/L = (D ÷ 2240) ÷ (0.01 x LWL)³
- D: Displacement of the boat in pounds.
- LWL: Waterline length in feet
Comfort Ratio
This ratio assess how quickly and abruptly a boat’s hull reacts to waves in a significant seaway, these being the elements of a boat’s motion most likely to cause seasickness. Read more.
Comfort ratio = D ÷ (.65 x (.7 LWL + .3 LOA) x Beam 1.33 )
- D: Displacement of the boat in pounds
- LOA: Length overall in feet
- Beam: Width of boat at the widest point in feet
Capsize Screening Formula
This formula attempts to indicate whether a given boat might be too wide and light to readily right itself after being overturned in extreme conditions. Read more.
CSV = Beam ÷ ³√(D / 64)
The 10M came with several different engine installations. Most were powered by the Atomic Four gas engine. Diesels were optional with a Faryman 25hp in 75, a Westerbeake 20hp diesel in 76. the Faryman again in 77, and a Volvo 23hp (MD11) from 78-80. Available with a taller rig: I(IG): 46.00’ / 14.02m J: 14.20’ / 4.33m P: 40.50’ / 12.34m E: 11.00’ / 3.35m SA %100: 549.35 sq.ft. /51.03m2
Embed this page on your own website by copying and pasting this code.

- About Sailboat Guide
©2024 Sea Time Tech, LLC
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pearson Yachts Portal

The Pearson 10M : 1973–1980
33 feet of company talent coming together in a boat that has been designed and executed to fill a specific role.
Pearson 10M
A new boat means as much to us as it does to you. To you it represents the culmination of a dream, escape, family fun afloat and, often, a trophy case full of awards that you've earned together.
To us a new boat is tangible evidence of our capabilities and integrity. It is our reputation; to be admired, evaluated and judged.
In this case, it's the new Pearson 10M. 33 feet of company talent coming together in a boat that has been designed and executed to fill a specific role, viz: to offer more boat for the money in 33 feet than anyone could imagine possible in today's inflationary economy at absolutely no sacrifice in Pearson quality standards.
Step aboard. Study the cockpit and deck layout. Uncluttered. Efficient. Safe. Observe the two sail lockers and lazarette. Now sit at the helm or if she's equipped with a wheel, stand behind it and check the visibility. The low profile cabin puts the world in full view without sacrificing below-decks headroom. Jump up and down if you like. That solid feeling under foot is Pearson quality fiberglass construction.
A quick tour around deck shows off a few other reasons why Pearson is the standard of excellence. Check the non-skid pattern, windows, handrails, hardware installations and locations. Look closely at the deck to hull fit and consider that no less than three fastening methods are used to insure a permanent, integral bond.
Now look below and see how to maximize a 29'2" waterline and an 11' beam. Here's all the space and efficiency you'll every need for entertaining, cruising offshore or proving the 10M's mettle on the race course. Standing at the foot of the ladder facing forward, the first reaction is "WOW! How did they do it?". To starboard is the galley, ice chest, range, sink , counter space and plenty of food stowage. Efficient is the word. Reach out and touch everything. To port (Any how many times do you see this in a 33' boat?) is a big quarterberth and navigation command center. Lift top chart table and plenty of access for all the electronics you'll every want. A few steps forward and you're in the center of the main salon. To port is a pull-out double berth and to starboard are upper and lower berths.
Now try the foldaway drop-leaf table. If it's down when you come aboard, one finger raises it to its foldaway position against the forward bulkhead adjacent to the mast. If it's tucked away, simply back off the lone wing-nut and gently lower it into position. Voila! Teak bookshelves where the table used to be. Ingenious. Look overhead. A big main cabin hatch (optional) for plenty of light and ventilation.
The toilet room is next; and who says "it's just a necessity." Check how it's made to facilitate cleaning; and the way the forward bulkhead curves to provide easy access forward at little sacrifice to interior space. Two hanging lockers are to starboard, and now you're in the spacious forward cabin. Two berths, stowage above, optional V filler to make a big double and anchor rode stowage forward.
The total impression is one of comfort, efficiency and maximum utililization of space. Fabrics, countertops and bulkheads are color coordinated to provide a rich, handsome, enlightened decor. Just add you personal touch to maker her YOUR boat.
We're obviously quite proud of our new member of the fleet and want you to be too. In the final analysis, that's what counts.
Pearson Yachts Inc. 1973

The boat design by Bill Shaw was built by the American company, Pearson Yachts, who are no longer in business, at their Rhode Island, United States plant.
In most ways the 10M fits nicely into the Pearson cruiser/racer approach reaching perhaps just a little closer to the racer end of the spectrum than other Pearson's of it's day. Production started in 1973 and ran until 1980 with about 236 boats produced. Peak production was in 1974 and 1975 and tapered off from there with only about a dozen boats being built in the last two years.
Pearson 10M – By The Numbers
Specifications*.
| LOA (Overall Length) | 33' |
| LWL (Waterline Length) | 28.3' |
| Beam | 11.0' |
| Draft | 5.9' |
| Displacement | 12,441 lbs |
| Ballast | 5,445 lbs |
| Sail Area | 524 ⁄ 549 sq ft |
| Mast Height (above D.W.L.) | 48' |
| Auxillary Power | Inboard |
| Foretriangle Area | 312 ⁄ 327 sq ft |
| Mainsail Area | 212 ⁄ 223 sq ft |
| I – Foretriangle Height | 44' ⁄ 46' |
| J – Foretriangle Base | 14.2' |
| P – Mainsail Hoist | 38.5' ⁄ 40.5' |
| E – Mainsail Foot | 11' |
| *approximate by Pearson Yachts Inc. |
Technical Data
| Designer | Bill Shaw |
| Years Built | 1973 – 1980 (236) |
| Hull Speed | 7.13 kn |
| SA/D – Sail Area to Displacement | 15.6 ⁄ 16.4 |
| DLR or D/L – Displacement to Length Ratio | 245 |
| BR – Ballast Ratio | 44% |
| L/B – Length to Ballast | 3.00 |
| LWL/B – Waterline Length to Ballast | 2.65 |
| OR – Overhang Ratio | 14% |
| CSF – Capsize Screening Formula | 1.90 |
| MCR – Motion Comfort Ratio | 25.8 |
| M/F – Main to Foretriangle Ratio | 0.68 |
| PHRF (avg) – Performance Handicap Rating | 141 ⁄ 135 |
Specifications
Hull: One-piece molded reinforced fiberglass laminate (hand lay-up) with integrally bonded bulkheads. Standard hull color white (with other colors optional). Cove stripe: standard colors. Boot top and bottom paint. .
Deck and Cockpit: One-piece reinforced fiberglass laminate (hand lay-up with balsa core for stiffness and insulation. Color and Non-skid surfaces molded in. Textured fiberglass full headliner laminated to deck cabin interior surface. Self-bailing cockpit. Cockpit sail lockers (P&S), with molded drain gutters. Lazarette hatch. Fiberglass sliding companionway hatch. Drop slides. Fiberglass coamings and winch islands with storage alcoves under. Teak handrails. Deck unit mechanically fastened to hull with overlay of fiberglass for complete watertight integrity. Choice of standard deck colors.
Machinery: 30H.P 4-cylinder gas engine. Direct drive, 35 amp. alternator. 7/8" Tobin bronze shaft, shaft strut, propeller, and bronze gland stuffing box. Water box muffler with steamhose exhaust line. Controls and instruments inside of cockpit well include shift, throttle, choke, starter button, ammeter, oil pressure, and water temperature. 20 gal. Monel fuel yank with cockpit sole deck plate fill and overboard vent. Shut –off valve, fuel filter, and flexible fuel line.
Tanks and plumbing: Two 20 gal. Fiberglass water tanks located in main cabin under (P&S) berths, with deck fills, vents, and supply lines with shut-off valves. Toilet intake line fitted with bronze seacock. Holding tank standard. Flush through hulls.
Electrical: Fused switch panel. Master power switch. Two 12V 90 AH batteries. International navigation lights. Chart table and interior cabin lights. Stranded copper wiring with impervious covering, color coded for circuit identification and located high above bilge area. Designed to minimize voltage drops
Hardware and deck Fittings: Chrome-platted brass or bronze, stainless steel, and special marine alloys, including custom designed stainless steel steamhead fitting, stainless steel backstay and shroud cabinplates. Bow chocks (P&S), bow cleat, stern cleats (P&S), and flagpole socket. Two large fixed ports (main cabin), and 4 small fixed ports. Dorade box with cowl vent over toilet room on portside cabintop. Transparent overhead hatch, forward cabin. Fordeck plate. Stainless steel genoa tracks through bolted on teak battern (P&S). Genoa blocks with track slides. 2 No. 43 Lewmar sheet winches (chrome) with cleats. Recessed main sheet traveler on bridge deck. Stainless steel stanchions with vinyl-covered stainless steel lifelines. Laminated tiller.
Spars: Tapered mast of protective coated 6060-T6 alloy with internal track section, stepped through the deck. Aluminum spreaders. Custom masthead fitting and stainless steel tangs. Halyard cleats. 2 No. 8 lewmar halyard winches (chrome). Boom of anodized aluminum 6061-T6 alloy with internal sail track groove. Fixed gooseneck. Internal block and tackle clew outhaul. Topping lift. Mainsheet and blocks.
Rigging: Standing stainless steel 1x19 with truloc swaged end fittings. Turnbuckles for headstay, backstay, upper and lower shrouds. Toggles on upper-lower shrouds and masthead toggles on headstay. Welded jiffy reef hooks on gooseneck.
Running Rigging: Main and Jib halyards of 7 x 19 stainless steel with spliced Dacron* tails. Braided Dacron* main and genoa sheets. Flag halyards.
Interior: Sleeping accommodations for seven. V-Berth in forward cabin with trap stowage under. Hanging locker to starboard. Forepeak anchor rode stowage. Translucent hatch overhead forward. Molded in shelf stowage (P&S). Hinged door privacy. Toilet room has fiberglass sole and toilet base. Alcoves and sliding door locker outboard. Fiberglass vanity equipped with manual F.W. pump, trap linen stowage, and locker under. Mirror with light over. Hinged entry door. Large hanging locker is opposite toilet room. Main cabin has pull-out double berth to port and pilot and transom berth to starboard. Built-in storage over port berth. Bulkhead mounted folding table with hinged lewars of high pressure laminate. Quarterberth and navigation station (with chart storage) is located aft to port; drawer and locker under. Stowage shelf outboard above quarterberth. Tack companionway ladder. L-shape galley aft to starboard. Large stainless steel galley sink with F.W. foot pump. Large top-loading icebox with foam in-place insulation. 2-burner countertop alcohol stove, gimbaled. Large sliding door locker outboard of galley countertop. Drawers and lock stowage under. Weather gear stowage opposite galley next to quaterberth. Textured fiberglass sole in both cabins. High pressure laminates on all countertops. Aft cabin face has hinged access panels (P&S) for instrument installations. Main bulkheads are covered with high pressure laminates. Hull sides are covered with veneer genuwall. Standard berth mattresses are 4" foam; fabric covered throughout. Teak trim and joiner work throughout. Choice of contemporary interior décor colors. Teak handrails overhead.
Safety equipment: Bonding system incorporates common grounding of chainplates, seacocks, engine and fuel tank. Seacock or gate valve on thru-hulls. Deck and cockpit areas have molded-in non-skid surfaces. Self-bailing cockpit. Natural and forced draft ventilation of engine compartment in accordance with U.S.C.G regulations. Manual bilge pump. Life lines. Teak handrails on cabin top. Teak interior handrails. Automatic fuels shut-off valve.
Optional equipment: A wide selection of factory installed equipment is available for this boat. See price sheet for complete details.

| Website | |
| Website | |
| Website |
Pearson 10M Brochure
Click to enlarge


- Forums New posts Unanswered threads Register Top Posts Email
- What's new New posts New Posts (legacy) Latest activity New media
- Media New media New comments
- Boat Info Downloads Weekly Quiz Topic FAQ 10000boatnames.com
- Classifieds Sell Your Boat Used Gear for Sale
- Parts General Marine Parts Hunter Beneteau Catalina MacGregor Oday
- Help Terms of Use Monday Mail Subscribe Monday Mail Unsubscribe
Why'd you buy a Pearson?
- Thread starter Dave C - pearsonowners.co
- Start date Dec 18, 2007
- Brand-Specific Forums
Dave C - pearsonowners.co
No tug boat The P-34 is no tug boat. I like her because she sails well, will move right along. A lot of these have been club racers. I was attracted by getting a good turn of speed and the legendary Pearson brick s___house construction. Being a mid eighties boat, there is good headroom below, decent beam w/o being a real tub. She's not the prettiest girl at the dance, but she sure can dance.
Sandy Stone
What he said I have a P-32, and agree with everything Shorty said. I would also add that for the amount of boat you get, the prices are generally very reasonable. One reason for this, I think, is the relatively simple and easy to maintain systems, which is just what I want on an older boat.
Dave C20469
No offense taken I was mostly surprised by the comment (tug), because my P-34 moves right along. Also second on Sandy's comment. When looking, my budget was 50 - 100K & I bought @ 50 which was good because, just like the rest of you, I proceeded to spend an irrational amount on upgrades, improvements, and other absolutely, cannot sail safely without, stuff. Comparable Sabres & Tartans were +20-40K.
Why I bought a peason Ive owned 2 pearsons over the last 25 years a 10 M and my current boat A p26w Pearsons have stood the test of time. They sail well and they are bullet proof Ive sailed on dozens of other makes lots are OK but in my opinion they just do not measure up to the venerable pearsons. If I could buy a brand new P26 today I would do It in a heartbeat. But I cant they just dont make them like that anymore. Ive completely restored MY 1975 26w to like new condition and Im sure it will still be sailing long after Im done.
Rich (P303)
Maine... No story... Looked through Yacht World for about 2 years... as well as over 50 boats. It was quite frankly the most boat for the buck. I am from the Black Sheep side of the family... not cut from the same cloth as most yachters. Beer budget. First choice would have been a Sabre. Lobster buoys... lobster buoys... lobster buoys... and built like a tank. Went for the P303's modified keel... to shed the traps. Yanmar FWC engine. In mast furler (probably one in a kind... and over-kill). 6'+ headroom. Wide beam. Short draft. Modest price. Roomy. Quality built. Not the prettiest girl on the block... but a great coastal cruiser. Only drawback... a lot of windage.
Why a Pearson? We've owned two -- first a P26, and after a bout of four-foot-itis, now we have a Pearson 30. We were looking for value for the buck (we're of limited means) and something that would give reasonable performance. You have that in the "two-digit" Pearson models like the 26, 30, 32, and 34 -- none of those are tugs! The 303 and the 323 were built less for racing and more for cruising with their low angle of heel and more commodious configurations. You give up performance for amenities, and as long as that's what you want, it works. We've done pretty decent racing the P30 here in Maine. Years ago, a sistership was quite competitive on the race course. We also cruise her for a couple weeks in the summertime. Not sure what the next boat will be. We're a little leary of the cored hull on the 34, and are not enamoured with the cabin-top mainsheet. Otherwise, we like the 34 a lot. We'll see.
Did it twice Had a Pearson 26 for 12 years and would have kept it except the kids got too big to cruise without "issues" (teenage girls). Bought a 303 and have had it for 9 years. The girls are now in college and working on weekends, so I'm thinking about another 26--my crew is usually my wife and our 9 year old golden retriever who has some "issues" of his own with the companionway ladder in the 303. I do almost all my own work and on 2 boats with a combinded age older than me that is a lot of systems to rebuild. The nice thing about Pearsons is you feel like all the time and effort is worth it--they are built well in the first place and the things that wear out can be fixed without tearing the boat completley apart (ususally). Both of my decisions were based on a cost/quality analysis and Pearson is the sweet spot on that continuim for me. Not to metion they look good, sail good and are fairly well respected on the waterfront. Sure I would like a Hinckley, but as Practical Sailor ssid in its review of the 303 "What's not to like?" It's the same ocean, either way. First post for me, looks like a good site. Glad to see some new 303 owners out there, you won't be sorry. I have done the hoses, head, water and holding tanks, added additional water under the v berth, ports, cabin sole, exhaust elbow, cutlass bearing, hot water tank, etc. so maybe I can help out. Don't worry, the boat would still sail without doing all this work, but it is nice to have things the way you want them. Here is a link to my boat. Would consider a P 26 in southern New England in partial trade. Best , Bob
P-34 cored hull Gail - when looking for a boat, one my no-no's was a cored hull & I ended up buying it anyway because it is such a solidly built boat. A cored hull in a Catalina or Hunter would probably be the kiss of death. I guess only time will tell. Midboom mainsheet cannot be reached from the helm but keeps the cockpit clear. Autopilot is a necessity for singlehanding.
It's all a tradeoff We have not completely eliminated a cored-hull boat, just a little hesitant. In fact, there's a certain P-34 in our area that we'd snatch right up if the price was right. On the mainsheet, yeah it's all a tradeoff. My other half learned to sail on an Ericson 33 with a bridge-deck mainsheet and that's the configuration he likes. You get ease of trimming, but the tradeoff is that the dodger can only come back so far before interfering with the mainsheet.
1st Pearson I am considering the purchase of a 303 and have heard lots of good things. How about some of the items that could be better or I need to watch for? Would a boat built in 1983 be better than a boat built in 1985? Appreciate any feedback. Cappep
1st Pearson Cappep, Pick whichever of the two boats are in best condition. There were no differances in the boats when they were new. Pearson used the same laminates from W.R. Grace throughout the 70's to the 90,s. Others experimented in the 80's with lighter laminates with bad results and most returned to the Grace products. Dave
They didn't have... many changes through the production years. The earlier ones had more fiberglass in the interior... the later ones had more teak, more teak/holly floor... chart table. Find the one that looks the best... and go for it. Great coastal and weekend cruiser. It is not a blue water boat. $25,000-$30,000 would be a ball park price in "good" condition depending on the year. My P303 was in great condition when purchased... so I couldn't tell you where to look for problems. Good Luck, Rich
303 Grace Products hanls for the info. What is "Grace Products"?
Grace Products Cappep, What I ment by Grace products was that the glass mat (laminants), epoxy rein, hardners, curing agents, etc. were produced by W.R. Grace Corp. They were prety much the leader of fiberglass products at the time. Other boat manufacturers tried other products to reduce weight and cost with various success. Pearson only changed chemistry after extensive study and trials. Somewhere in my notes I have the name of the company that made the gel coating. At the time I thought it might be important for future repairs. Now, with new developments of fiberglass products, it no longer makes any differance as to the glass or gel coat manufacturer to do repairs. The point I was making is that Pearson developed a manufacturing method and suppliers that worked well and stayed with it. Dave s/v ARIEL
- This site uses cookies to help personalise content, tailor your experience and to keep you logged in if you register. By continuing to use this site, you are consenting to our use of cookies. Accept Learn more…
- New Sailboats
- Sailboats 21-30ft
- Sailboats 31-35ft
- Sailboats 36-40ft
- Sailboats Over 40ft
- Sailboats Under 21feet
- used_sailboats
- Apps and Computer Programs
- Communications
- Fishfinders
- Handheld Electronics
- Plotters MFDS Rradar
- Wind, Speed & Depth Instruments
- Anchoring Mooring
- Running Rigging
- Sails Canvas
- Standing Rigging
- Diesel Engines
- Off Grid Energy
- Cleaning Waxing
- DIY Projects
- Repair, Tools & Materials
- Spare Parts
- Tools & Gadgets
- Cabin Comfort
- Ventilation
- Footwear Apparel
- Foul Weather Gear
- Mailport & PS Advisor
- Inside Practical Sailor Blog
- Activate My Web Access
- Reset Password
- Customer Service

- Free Newsletter

Marshall Sanderling 18: Used Boat Review

Affordable Cruising Sailboats, Continued

Maine Cat 41 Used Boat Review

CS 30 Used Boat Review

Best Crimpers and Strippers for Fixing Marine Electrical Connectors

Thinking Through a Solar Power Installation

How Does the Gulf Stream Influence our Weather?

Can You Run a Marine Air-Conditioner on Battery Power?

Need a New Headsail Furler? Here’s What’s Involved

Master the Sailing Basics: Never Stop Learning the Little Things

How to Mount Your Camera on Deck: Record Your Adventures with…

Un-Stepping the Mast for America’s Great Loop

Sinking? Check Your Stuffing Box

The Rain Catcher’s Guide

How to Change Your Engine Mounts

Keeping Water Clean and Fresh

Vinyl Boat Lettering DIY Application and Repair

Those Extras you Don’t Need But Love to Have

Three-Model BBQ Test

Alcohol Stoves— Swan Song or Rebirth?

UV Clothing: Is It Worth the Hype?

Preparing Yourself for Solo Sailing


How to Select Crew for a Passage or Delivery

Preparing A Boat to Sail Solo

Dear Readers

Chafe Protection for Dock Lines

Waxing and Polishing Your Boat

Reducing Engine Room Noise

Tricks and Tips to Forming Do-it-yourself Rigging Terminals
- Sailboat Reviews
A Deck Level View of the Pearson Commander

Freeboard on the Commander is somewhat low (top), which sometimes makes for a wet, but exciting ride in bumpy conditions.
1. The generous cockpit featured on the Commander realistically seats six while under sail, but will accommodate more while at anchor with the tiller folded up. The cockpit is self-draining, but could use larger drains. Anyone with offshore aspirations will want to better seal the main hatch and lazarette.
2. If you need to move forward quickly in a seaway, perhaps to access the headsail, you will find the way there somewhat complicated. Shrouds occupy the narrow side decks, which necessitate shimmying sideways in- or outside them.
3. Try not to trip over one of the original bronze winches.
4. Like most well outboards, the one in the Pearson Commander requires an open hatch to ensure adequate air for proper operation.
RELATED ARTICLES MORE FROM AUTHOR
Leave a reply cancel reply.
Log in to leave a comment
Latest Videos

Pearson 37 & 37-2 – Behind the Curtain

How To Test a Boat Engine

Hunter Legend 35.5 – Behind the Curtain

Whipping Line On Your Sailboat
Latest sailboat review.

- Privacy Policy
- Do Not Sell My Personal Information
- Online Account Activation
- Privacy Manager
ORIGINAL RESEARCH article
Ph and thiosulfate dependent microbial sulfur oxidation strategies across diverse environments.

- 1 Department of Civil and Mineral Engineering, University of Toronto, Toronto, ON, Canada
- 2 Department of Earth and Planetary Science, University of California, Berkeley, Berkeley, CA, United States
- 3 School of Mathematical and Physical Sciences, University of Technology Sydney, Ultimo, NSW, Australia
- 4 Commonwealth Scientific Industrial and Research Organization, Black Mountain, ACT, Australia
- 5 EcoReg Solutions, Guelph, ON, Canada
- 6 Commonwealth Scientific Industrial and Research Organization, Clayton, VIC, Australia
Sulfur oxidizing bacteria (SOB) play a key role in sulfur cycling in mine tailings impoundment (TI) waters, where sulfur concentrations are typically high. However, our understanding of SOB sulfur cycling via potential S oxidation pathways ( sox , r dsr , and S 4 I) in these globally ubiquitous contexts, remains limited. Here, we identified TI water column SOB community composition, metagenomics derived metabolic repertoires, physicochemistry, and aqueous sulfur concentration and speciation in four Canadian base metal mine, circumneutral-alkaline TIs over four years (2016 – 2019). Identification and examination of genomes from nine SOB genera occurring in these TI waters revealed two pH partitioned, metabolically distinct groups, which differentially influenced acid generation and sulfur speciation. Complete sox (c sox ) dominant SOB (e.g., Halothiobacillus spp., Thiomonas spp.) drove acidity generation and S 2 O 3 2- consumption via the c sox pathway at lower pH (pH ~5 to ~6.5). At circumneutral pH conditions (pH ~6.5 to ~8.5), the presence of non-c sox dominant SOB (hosting the incomplete sox , r dsr , and/or other S oxidation reactions; e.g. Thiobacillus spp., Sulfuriferula spp.) were associated with higher [S 2 O 3 2- ] and limited acidity generation. The S 4 I pathway part 1 ( tsdA ; S 2 O 3 2- to S 4 O 6 2- ), was not constrained by pH, while S4I pathway part 2 (S 4 O 6 2- disproportionation via tetH ) was limited to Thiobacillus spp. and thus circumneutral pH values. Comparative analysis of low, natural (e.g., hydrothermal vents and sulfur hot springs) and high (e.g., Zn, Cu, Pb/Zn, and Ni tailings) sulfur systems literature data with these TI results, reveals a distinct TI SOB mining microbiome, characterized by elevated abundances of c sox dominant SOB, likely sustained by continuous replenishment of sulfur species through tailings or mining impacted water additions. Our results indicate that under the primarily oxic conditions in these systems, S 2 O 3 2- availability plays a key role in determining the dominant sulfur oxidation pathways and associated geochemical and physicochemical outcomes, highlighting the potential for biological management of mining impacted waters via pH and [S 2 O 3 2- ] manipulation.
1 Introduction
Biological sulfur oxidation can present significant risks to the environment via acid generation, contaminant mobilization, and oxygen consumption (i.e., acid mine drainage) ( Edwards et al., 1999 ; Elberling and Damgaard, 2001 ; Baker and Banfield, 2003 ; Druschel et al., 2003 ; Johnson and Hallberg, 2003 ; Korehi et al., 2014 ). This biological acidity production occurs in both natural (termed acid rock drainage, found in alpine catchments; Lacelle et al., 2007 ; Zarroca et al., 2021 ) and anthropogenic (e.g., mine tailings impoundments and waste rock piles; Akcil and Koldas, 2006 ) environments, though the scale to which it occurs in anthropogenic environments is typically much larger.
Base metal mine tailings impoundment (TI) wastewaters often have elevated sulfur concentrations due to the dominance of sulfide minerals in base metal ores [e.g., chalcopyrite (CuFeS 2 ), sphalerite ((Zn, Fe)S), etc.]. Sulfides can be partially oxidized during the grinding, flotation, and leaching steps of sulfide mineral ore extraction ( Liljeqvist et al., 2011 ), resulting in the production and subsequent release of sulfur oxidation intermediate compounds (SOI) from tailings streams to TIs. In addition to sulfide (ΣH 2 S), SOI commonly found in mining impacted waters include thiosulfate (S 2 O 3 2- ), tetrathionate (S 4 O 6 2- ), elemental sulfur (S 0 ), sulfite (SO 3 2- ), and a range of other polythionates (S x O y 2- ) ( Makhija and Hitchen, 1979 ; Miranda-Trevino et al., 2013 ; Whaley-Martin et al., 2020 ). The concentrations of individual SOI in TI wastewater can vary significantly spatially, seasonally, and amongst mining operations. SOI may be present in relatively high concentrations in tailings slurries, but are typically much lower in the TI waters due to dilution by other water inputs collected in these actively managed systems ( Thamdrup et al., 1994 ), though they are typically higher than concentrations observed in natural environments ( Foucher et al., 2001 ; Canfield and Farquhar, 2009 ; Silva et al., 2012 ; Camacho et al., 2020b ; Vincent et al., 2021 ). SOI can be reduced, oxidized, and disproportionated, resulting in differential SOI speciation and pH outcomes, via both abiotic and biotic reactions ( Philippot et al., 2007 ; Klatt and Polerecky, 2015 ; Houghton et al., 2016 ) further contributing to the complexity of the sulfur cycle in these environments. As mine TI systems continue to grow in number and size around the world, an understanding of the biogeochemical cycling of sulfur compounds occurring within these contexts, the SOB involved, and the influencing factors determining water quality outcomes is increasingly important.
Few studies have addressed the coupling of sulfur oxidation metabolic pathways in TIs to SOB taxonomy, physicochemistry, and sulfur geochemistry ( Whaley-Martin et al., 2019 , 2023 ; Miettinen et al., 2021 ), contrasting more well-studied extremophilic acid mine drainage environments ( Bond et al., 2000 ; Johnson and Hallberg, 2003 ; Dold, 2014 ). Recent research has highlighted a divergence of microbial communities found in circumneutral mining impacted TI waters from those of acid mine drainage environments ( Whaley-Martin et al., 2019 , 2023 ; Camacho et al., 2020b ; Lopes et al., 2020 ; Miettinen et al., 2021 ). A number of sulfur oxidation pathways have been identified as being used by SOB potentially found in mining environments, though the physicochemical, geochemical, and/or ecological parameters governing which S oxidation pathway(s) occur are not well defined ( Han and Perner, 2015 ; Wang et al., 2016 ; Watanabe et al., 2019 ). These pathways include the sulfur oxidation ( sox ), reverse dissimilatory sulfite reductase (r dsr ), and tetrathionate intermediate (S 4 I) pathways ( Friedrich et al., 2001 ; Han and Perner, 2015 ; Klatt and Polerecky, 2015 ; Wang et al., 2016 ; Watanabe et al., 2019 ; Whaley-Martin et al., 2023 ). The sox pathway has seven structural genes which encode four proteins (soxXA, soxYZ, soxB, and soxCD) allowing this pathway to mediate S 2 O 3 2- , SO 3 2- , S 0 , and ΣH 2 S dependent cytochrome c reduction ( Friedrich et al., 2001 ). SoxAX catalyzes the attachment of S 2 O 3 2- to cysteine residue on the soxY of the soxYZ complex (forming soxZY-Cys-S - ) ( Friedrich et al., 2000 , 2001 ; Bamford et al., 2002 ; Watanabe et al., 2019 ). The soxCD complex then oxidizes the sulfate sulfur from SoxZY-Cys-S - to produce the sulfonate group as soxZY-Cys-SO 3 - ( Quentmeier et al., 2000 ; Zander et al., 2011 ; Watanabe et al., 2019 ). SoxB further hydrolyzes the sulfane sulfur from SoxZY-Cys-SO 3 - to a free sulfate ion ( Quentmeier et al., 2000 ; Zander et al., 2011 ; Watanabe et al., 2019 ). Free SOI are not produced by the sox pathway when found in its complete form (complete sox ; c sox ) as they are covalently attached to soxYZ until complete oxidation to sulfate (SO 4 2- ; Grabarczyk and Berks, 2017 ). An incomplete form of the sox pathway (lacking soxCD, i sox ) has also been identified which can form S 0 ( Frigaard and Dahl, 2008 ; Watanabe et al., 2019 ). This S 0 can be subsequently oxidized by r dsr, sulfur-oxidizing heterodisulfide reductase-like (s hdr ), or sulfur dioxygenase ( sdo ) to SO 3 2- ( Klatt and Polerecky, 2015 ). The r dsr pathway is composed of the same proteins as the dsr pathway ( sat , aprAB , dsrAB ) though the reductive and oxidative varieties are phylogenetically discernible ( van Vliet et al., 2021 ). SOI including S 2 O 3 2- , S 0 , and ΣH 2 S, can be oxidized through the r dsr pathway and generate free SO 3 2- ( Klatt and Polerecky, 2015 ). Sulfane sulfur generated from the i sox pathway can then be transported to the cytoplasm in the form of persulfides (R-S-S - ) to be further oxidized to HSO 3 - by dsrAB or s hdr ( Pott and Dahl, 1998 ; Frigaard and Dahl, 2008 ; Cao et al., 2018 ; Koch and Dahl, 2018 ). The HSO 3 - can be further oxidized to APS (adenosine 5’-phosphosulfate) by aprBA with electron transfer by aprM or hdrAACB and then to SO 4 2- by sat ( Meyer and Kuever, 2007 ; Loy et al., 2009 ). R dsr pathway presence is typically associated with high energy efficiency compared to the sox pathway ( Klatt and Polerecky, 2015 ). Several beta- and gammaproteobacteria have been found to utilize the S 4 I pathway (or Kelly-Trudinger pathway) which generates free S 4 O 6 2- ( Dam et al., 2007 ). The S 4 I pathway oxidizes S 2 O 3 2- to S 4 O 6 2- by thiosulfate dehydrogenase ( tsdA ) or thiosulfate:quinol oxioreductase ( TQO or doxD ; Brito et al., 2015 ; Wang et al., 2016 ; Hutt et al., 2017 ). Subsequent processing of free S 4 O 6 2- can be catalyzed by tetrathionate reductase ( ttrABC ) to produce S 2 O 3 2- or tetrathionate hydrolase ( tetH ) to produce S 2 O 3 2- , S 0 , and SO 4 2- , which are both common in SOB ( Wang et al., 2016 ; Camacho et al., 2020a ; Miettinen et al., 2021 ). A recent study ( Whaley-Martin et al., 2023 ) identified oxygen as a control on whether the c sox (high O 2 ) or r dsr (low O 2 ) pathway dominated in one TI with differing water quality outcomes, indicating that physicochemical and/or SOI substrate partitioning of SOB within TI may happen more broadly.
The objectives of this cross-mine study were to identify mining TI associated SOB, examine functional differences in SOB communities, and align their associated S oxidizing repertoires to geochemical and physicochemical characteristics and outcomes in circumneutral TI waters of four base metal mines located across Canada (Manitoba, Newfoundland, Ontario). A better understanding of these genetic, geochemical, and/or physicochemical connections will inform biological management strategies as well as further understanding of S biogeochemical cycling more broadly. Four years (2016 – 2019) of S geochemistry, physicochemistry, and genus level community structure and function data from the four base metal mine TIs were examined. To determine if the findings of this study were site specific or reflective of broader environmental trends, comparisons were made to published studies on other mines and industrial environments, as well as natural environments.
2 Materials and methods
2.1 site descriptions.
Four base metal mine TI waters were sampled from 2016 to 2019 resulting in a total of 42 water samples (see Supplementary Figure S1 ). These four mines are located across central and eastern Canada and consist of: Mine 1 in Flin Flon, Manitoba (Cu, Zn, Au, Ag), Mine 2 in Sudbury, Ontario (Ni, Cu, Co, Pt, Pd), Mine 3 in Snow Lake, Manitoba (Cu, Zn, Se, Te, Ag), and Mine 4 in Baie Verte, Newfoundland (Cu, Au). The mines range in size, age, and stage of development with Mine 1 and Mine 2 being the oldest (operating on and off since 1927 and 1928, respectively), Mine 3 opened in 1979 and Mine 4 as the youngest with mining originating on the property around 1997, with the commissioning of the TI in 2009 ( Table 1 ). The TI facilities across sites also vary in size and depth with Mine 2 being both the largest and deepest at ~38 m, followed by Mine 1 at ~7 to 10 m depth and finally Mine 3 and Mine 4 at ~1.5 to 2.5 m depth. TI water cover sampling depths for Mine 1, Mine 2, Mine 3, and Mine 4 ranged 5 to 10 m, 0.5 to 10 m, 0.5 to 3 m, and 0.5 to 1.5 m, respectively, and this investigation targeted seasonal open water collection (early spring to late fall).
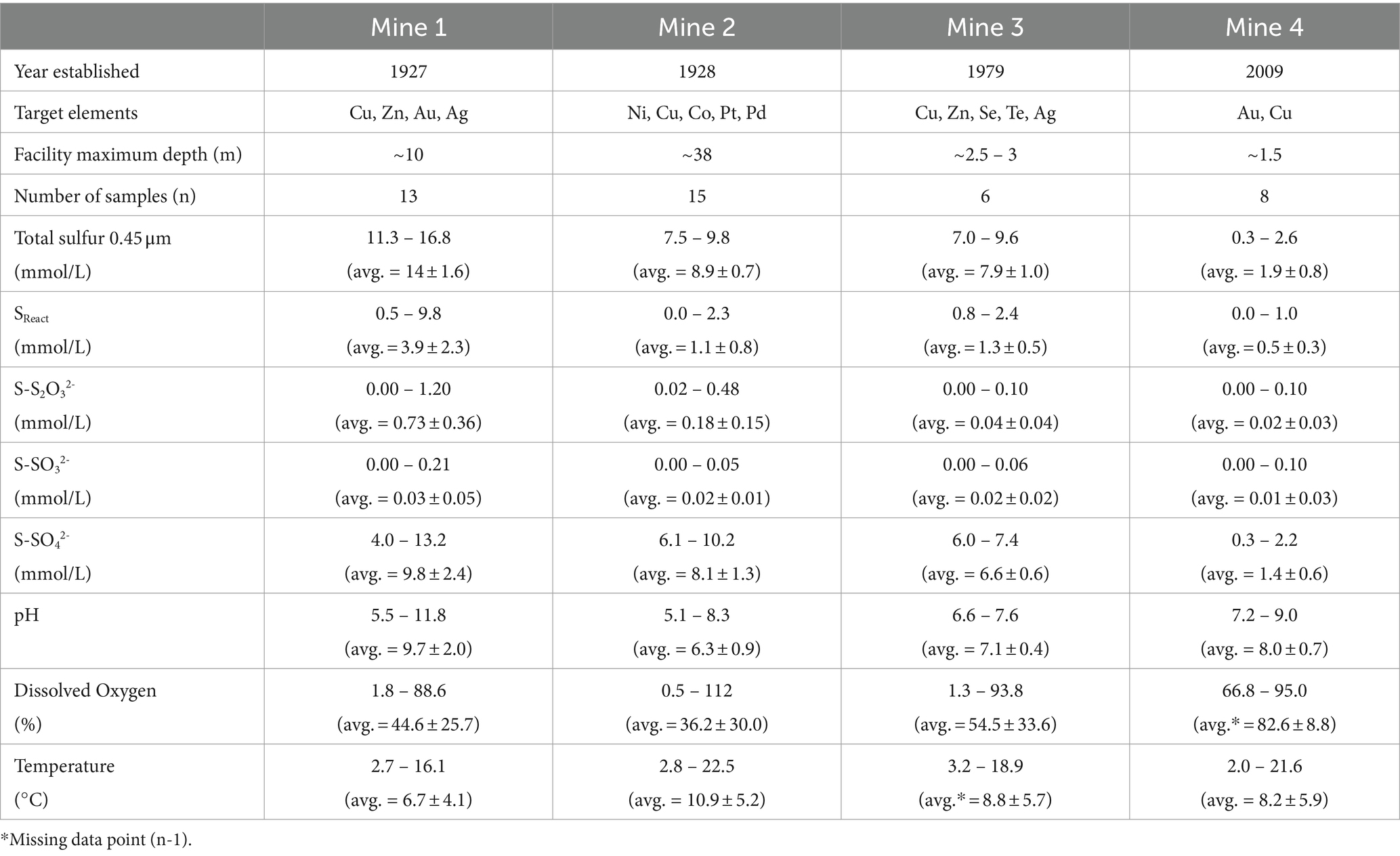
Table 1 . Mine information, sulfur geochemistry, and physicochemistry data for Mine 1, Mine 2, Mine 3, and Mine 4 tailings impoundment waters (2016 – 2019) presented as average (± standard deviation).
2.2 Physicochemical characterization and sampling scheme
Samples collected from Mine 2 TI were taken from two different points in the reservoir (see Supplementary Figure S2 ) which include a floating platform at the deepest point of the reservoir and the outflow dam. The outflow dam represents an approximate average of the overall water column and is ~2 m deep at the sampling location (samples taken at 0.5 m or 1 m). Samples from Mine 3 and Mine 1 TIs were collected off the end of docks ~4 m from shore. Mine 4 TI samples were collected from a boat ~20 m from shore.
Physicochemical and geochemical water samples from Mine 2 were collected and processed on-site within 1 h of collection, while water samples from Mine 1, Mine 3, and Mine 4 were shipped to the University of Toronto for processing, taking two to ten days potential shipping impacts addressed in Whaley-Martin et al. (2019) . Depth samples from Mine 1 and Mine 2 were collected using a sterilized Van Dorn sampler as described in Whaley-Martin et al. (2019) and Mine 3 and Mine 4 samples were collected using a sterilized surface grab sampler. Water samples from Mine 1 and Mine 3 were collected in polyethylene liners (Uline S-1379) that were 70% ethanol sterilized and rinsed with target sample water three times prior to filling, using water directly from the Van Dorn sampler or surface grab sampler. Once 10 – 20 L were collected, liners were sealed without headspace, and placed in clean 20 L containers for shipping. Containers and liners remained unopened until arrival at the laboratory at the University of Toronto. Mine 2 water sample collection for microbial analysis followed the same protocol. Mine 4 water samples were collected into 70% ethanol sterilized and thrice rinsed (with target sample water) polyethylene containers before being submerged and filled directly leaving no headspace.
Each TI water sample was characterized for sulfur geochemistry and microbial community composition. Geochemical characterization included analyses of total sulfur (Total S), sulfate (SO 4 2- ), thiosulfate (S 2 O 3 2- ), and sulfite (SO 3 2- ) concentrations and determination of reactive sulfur (S React ; all S atoms capable of oxidation; determined by [Total S] – [SO 4 2- ]; Whaley-Martin et al., 2020 ). Microbial community structure and function assessment included 16S rRNA and metagenomic analyses. Field measured TI water physicochemical parameters including temperature, pH, dissolved oxygen (DO; concentration and % saturation) and conductivity or salinity data were collected using a YSI 600 XLM (Mine 2), ProDSS water quality meter (Mine 1 and Mine 3), or a ThermoScientific Orion Star A329 Multiprobe (Mine 4).
Samples collected for 16S rRNA and metagenomic analyses were vacuum filtered (~2 to ~5 L) in triplicate through 0.1 μm and 0.2 μm filter units (Thermo Scientific™ Nalgene™ Rapid-Flow™ Sterile Disposable Filter Units with aPES Membrane) until clogged. Filters were immediately excised in a sterile Biological Safety Cabinet and kept frozen at -80°C until future extraction.
2.3 Geochemical analyses
Total sulfur samples were collected in triplicate by filtering 40 mL (per replicate) through a 0.45 μm filter (Pall Acrodisc ® 25 mm 0.45 μm Supor ® membrane filters) with polypropylene syringes into 50 mL Falcon™ tubes pre-spiked with 80 μL of HNO 3 (Optima grade, Fisher Chemical). Unfiltered (UF) and 0.2 μm filtered total S samples were also collected, however, one-way ANOVA analysis revealed no statistically significant difference between the three filter fractions ( p = 0.998) from these TI waters, therefore the 0.45 μm filter fraction data were used as these values represented the most complete dataset. These samples were stored at 4°C until they were shipped to the Commonwealth Scientific and Industrial Research Organization (CSIRO) for analyses using an inductively coupled plasma atomic emission spectroscopy (ICP-AES) on a Varian730 ES (Mulgrave, Australia). The limit of detection (LOD) for sulfur was 0.03 mM and concentrations were calculated by measuring intensity at the 181.972 nm sulfur emission line. Fast Automated Curve-fitting Technique (FACT) was used to correct for background and inter-element interferences.
100 μL aliquots of sample waters were preserved in triplicate for S 2 O 3 2- and SO 3 2- analysis using scaled derivatization methods described in Rethmeier et al. (1997) . Samples were frozen until analysis. [S 2 O 3 2- ] and [SO 3 2- ] were quantified using a Shimadzu LC-20 AD prominence liquid chromatography (LC) system coupled with a fluorescence UV/VIS detector. An Alltima™ HP C18 reversed phase column (150 mm × 4.6 mm × 5 μm, Grace™) was used at 35°C using an isocratic mobile phase comprising 35% HPLC grade methanol and 65% of 0.25% acetic acid v/v (filtered, pH adjusted to 3.5 using NaOH) at 1 mL/min. The total run time was 12 min and the SO 3 2- peak eluted at ~3.1 min and S 2 O 3 2- eluted at ~3.4 min. The excitation wavelength used was 380 nm and the emission wavelength was 478 nm. Calibration curves were prepared using Na 2 SO 3 (Sigma Aldrich, ≥ 98% purity) and Na 2 S 2 O 3 (Sigma Aldrich, 99% purity). Limits of quantification for both S 2 O 3 2- and SO 3 2- were 0.01 mM.
Prior to 2019, SO 4 2- samples were collected into clean sample bottles that were thrice rinsed (with target sample water) and filled leaving no headspace. Samples were stored at 4°C until analyzed. Aqueous dissolved SO 4 2- was quantified by spectrophotometry using a HACH DR2800 (HACH Company, Loveland, CO, United States) using USEPA SulfaVer 4 Method 8051. SO 4 2- samples collected in 2019 were 0.2 μm filtered (Pall Acrodisc ® 25 mm 0.2 μm Supor ® membrane filters) in triplicate using polypropylene syringes into 1.7 mL polypropylene vials and stored at 4°C until analyzed. Waters were analyzed for anions following USEPA Method 300.0/300.1 (only SO 4 2- is reported here) on a Dionex™ ICS-6000 Capillary HPIC™ (high pressure ion chromatography; Thermo Scientific™, Part Number: ICS6000-003) System with a Dionex™ ICS-6000 CD Conductivity Detector (Thermo Scientific™, Part Number: 079829) and a Dionex IonPac™ AS18-Fast, 4 × 150 mm AS18 anion-exchange column (Thermo Scientific™, Part Number: 072062) and a Dionex IonPac™ AG18-Fast, 4 × 30 mm guard column (Thermo Scientific™, Part Number: 075762). A Dionex™ ICS-6000 EG Eluent Generator was used to produce the isocratic mobile phase of 23 mM KOH. Sample injection volume was 10 μL with sample aliquots diluted using 18.2 MΩ·cm deionized water when required. Matrix spikes and check standards were run every 20 samples and were also expected to fall withing ±15% of the expected value. The limit of detection for these samples was determined to be 0.008 mM dissolved SO 4 2- with an instrument error of ±0.1 mM ( Whaley-Martin et al., 2020 ; Yan et al., 2022 ).
Previous comparisons of the HACH spectrophotometry and IC methodology were completed on these waters by Whaley-Martin et al. (2020) identifying that dissolved [SO 4 2- ] obtained by HACH spectroscopy and IC methods were consistent between methods for measured dissolved [SO 4 2- ] between 0 and 250 mg/L.
2.4 16S rRNA sampling and statistical analyses
Genomic DNA was extracted from filters using Qiagen DNEasy PowerWater DNA Isolation Kits and the sample extracts were submitted to the McMaster University Genome Facility (Hamilton, Ontario, Canada) for subsequent analysis. Genomic DNA was quantified using quantitative PCR (polymerase chain reaction). Aliquots of purified DNA were used to amplify the V4 region of the 16S rRNA gene following the methods from Bartram et al. (2011) using Illumina primers and standards protocols from the Earth Microbiome Project ( Caporaso et al., 2011 , 2012 ). Briefly, the primers amplified the 515f (5′-GTGYCAGCMGCCGCGGTAA-3′) and 806r (5′-GGACTACNVGGGTWTCTAAT-3′) V4-variable regions of bacterial and archaeal 16S rRNA gene. 50 ng of DNA template was used for PCR and the PCR mix contained 1 U of recombinant Taq DNA Polymerase (Invitrogen™), 1x buffer, 1.5 mM MgCl 2 , 0.4 mg/mL bovine serum albumin (BSA), 0.2 mM deoxynucleotide triphosphate (dNTPs) and 5 pM of each primer. In accordance with Bates et al. (2010) , the PCR reaction was as follows: initial denaturing at 98°C for 5 min, 35 cycles of denaturing at 98°C for 30 s, annealing at 50°C for 30 s and an extension at 72°C for 30 s with a final extension at 72°C for 10 min. The PCR products were confirmed using electrophoresis and sent for sequencing. A SequalPrep normalization kit (ThemoFisher #A1051001) was used to normalize all amplicons to 1.25 ng/L and then sequenced using the Illumina Mi-Seq.
DADA2 (v. 1.6.0) was used to check data for bimeras; 3 – 5% of the reads were determined to be bimeras and excluded from the dataset. Sequences which have undergone DADA2 denoising are referred to as amplicon sequence variants (ASVs). Cutadapt was used to filter and trim raw sequences using a minimum read length of 100 bp and used a minimum quality score of 30 ( Martin, 2011 ). ASV tables were then merged and combined for each Illumina run and the SILVA database (v. 138.1) was used for taxonomic assignment using RStudio v. 1.4.1. While 16S rRNA calculated relative abundance is a widely employed method for assessing microbial community composition (e.g., Seth et al., 2024 ; Yu et al., 2024 ) due to its decreased cost, speed, and scalability ( Durazzi et al., 2021 ), it is important to acknowledge potential sources of error inherent in this approach. These potential sources of errors include variability in 16S gene copy number and amplification/sequencing bias and error ( Suzuki and Giovannoni, 1996 ; Hong et al., 2009 ; Kembel et al., 2012 ). However, their combination with other independent lines of evidence, i.e., metagenome and geochemical data, can assist in robust understanding of microbial community composition, function, and environmental preferences ( Louca et al., 2018 ; Campa et al., 2022 ; Whaley-Martin et al., 2023 ).
Shannon Diversity index and sample richness values were calculated in RStudio v. 1.4.1 based on the number of unique amplicon sequence variant (ASVs) and sequence abundances. Data visualization and linear regression (Pearson’s correlations and associated ANOVA tests) were performed in OriginPro v. 2022. Additional ANOVA and post-doc Tukey pairwise statistical analyses were performed in RStudio v. 1.4.1.
2.5 Metagenomic sequencing, reads processing, and assembly
The genomic DNA was extracted from the sample filters individually and used for subsequent metagenomic sequencing. The DNA extracts were dried and resuspended in 25 μL aliquots of water. The Illumina library preparation kits were used in the construction of sequencing libraries with an insert length of ~500 bp. The libraries were sequenced using the Illumina HiSeq-1500 platform with paired-end 150 bp sequencing kits by the Farncombe Metagenomics Facility at McMaster University (Hamilton, Ontario, Canada), as previously described in Whaley-Martin et al. (2023) .
The raw sequencing reads were filtered to remove Illumina adapters, PhiX and other Illumina trace contaminants using BBTools ( Bushnell et al., 2017 ) and low quality bases and reads were removed using Sickle (v. 1.33). All reads with both ends remained were used for subsequent de novo assembly via IDBA_UD (parameters: --mink 20, --maxk 140, --step 20, --pre_correction; Peng et al., 2012 ) or metaSPAdes (parameters: -k 21,33,55,77,99,127; Nurk et al., 2017 ). Sequencing coverage for each scaffold from a given sample was individually mapped using quality paired-end reads to the full assembly using Bowtie2 with default parameters ( Langmead and Salzberg, 2012 ). Generated sam files were converted to bam format and sorted using samtools ( Li et al., 2009 ). Then the coverage of each scaffold was calculated using the script of jgi_summarize_bam_contig_depths from MetaBAT ( Kang et al., 2015 ). Using MetaBAT, all scaffolds with a minimum length of 2,500 bp were assigned to genome bins with both tetranucleotide frequency and sequencing coverage profiles from all samples considered. Both binned and unbinned scaffolds with a minimum length of 1,000 bp were uploaded to ggKbase 1 for manual genome bin refinement, which was based on GC content, sequencing coverage, and taxonomic information of each scaffold as previously described in Chen et al. (2020) and was primarily based on patterns and distribution of GC content and sequencing coverage of contigs in each bin. Contamination contigs/scaffolds were removed from metagenome assembled genomes using ggKbase if the contig/scaffold had divergent sequencing coverage (those with <0.5x or > 2x coverage relative to most other contigs/scaffolds in the metagenome assembled genome), GC content, and/or divergent taxonomic assignment. Taxonomic assignment of metagenome assembled genomes was determined based on taxonomic classification of protein-coding genes within each contig or scaffold. This process involved identifying the best taxonomic matches of the protein-coding genes to references proteins and determining the last common ancestor where ≥50% of the matches align with a particular taxonomic group. Overall taxonomic assignment of a metagenome assembled genome was then derived from the cumulative taxonomic assignments of all its contigs/scaffolds.
2.5.1 Gene prediction and annotation
Gene prediction and subsequent analyses were completed using assembled scaffolds with a minimum length of 1,000 bp (herein “1k_scaffolds) to assist in obtaining metagenome assembled genomes with minimal contamination and high completeness. The protein-coding genes were predicted using Prodigal v. 2.6.3 from 1k_scaffolds (parameters: -m -p meta; Hyatt et al., 2010 ). 16S rRNA genes were predicted from 1k_scaffolds based on an HMM database in accordance with methods presented by Brown et al. (2015) . The tRNAs on all 1k_scaffolds were predicted using tRNAscanSE (v. 2.0.3; Chan and Lowe, 2019 ). For functional annotation, the predicted protein-coding genes were searched against the Kyoto Encyclopedia of Genes and Genomes databases (KEGG; Kanehisa et al., 2017 ), UniRef100 ( Suzek et al., 2007 ), and UniProt ( Apweiler et al., 2004 ) via Usearch (v.10.0.240_i86linux64; Edgar and Bateman, 2010 ). HMM databases ( Anantharaman et al., 2016 ) and KOfam HMM database ( Kanehisa and Sato, 2020 ) were used to search and identify the predicted protein-coding genes for specific metabolic potentials of interest. DiSCo ( Neukirchen and Sousa, 2021 ) was used to distinguish dsrC and its homologs. BLASTp was used to search predicted proteins against the shdr protein sequences encoded by Acidithiobacillus caldus SM-1, Thioalkalivibrio sp. K90mix, and Hyphomicrobium denitrificans ( Koch and Dahl, 2018 ) followed by manual confirmation. Screening for hdrAACB genes was completed by checking the annotations from KEGG, UniRef100, and UniProt using the key word “hdr” and the acquired list of genes was manually confirmed. The aprM and aprBA genes were identified by comparing all protein-coding genes (from corresponding metagenomes) against aprM / aprBA identified in Thiobacillus denitrificans (WP_011312796.1) using BLASTp with an e-value threshold of 1e-10 and hits were manually verified. The tetH gene was identified using a BLASTp search as previously described in Watanabe et al. (2019) followed by manual verification. Failure to detect genes which require annotated reference genomes (s hdr, hdrAACB, aprM, aprBA , and tetH ) for detection, rather than KO values (or other database values), may result in under-reporting of those genes until more annotated reference genomes are reported in the literature.
3 Results and discussion
3.1 sob community 16s rrna composition and metagenomic inferred function.
Microbial community composition was determined using 16S rRNA relative abundances for each TI sample ( n = 13 from Mine 1, n = 15 from Mine 2, n = 6 from Mine 3, and n = 8 from Mine 4). Nine major genera of SOB were identified in these 42 TI samples including: Halothiobacillus spp. (12.7 ± 20.5%), Sediminibacterium spp. (4.8 ± 9.1%), Thiobacillus spp. (3.9 ± 7.8%), Sulfuricurvum spp. (3.7 ± 11.5%), Thiovirga spp. (1.5 ± 3.2%), Sulfuritalea spp. (0.7 ± 2.9%), Sulfurimonas spp. (0.4 ± 1.3%), Sulfuriferula spp. (0.4 ± 0.7%), and Thiomonas spp. (0.1 ± 0.2%). Each genus occurred at >1% abundance in at least one sample ( Table 2 ). The presence and relative abundance of these nine SOB genera differed among mines and over time.
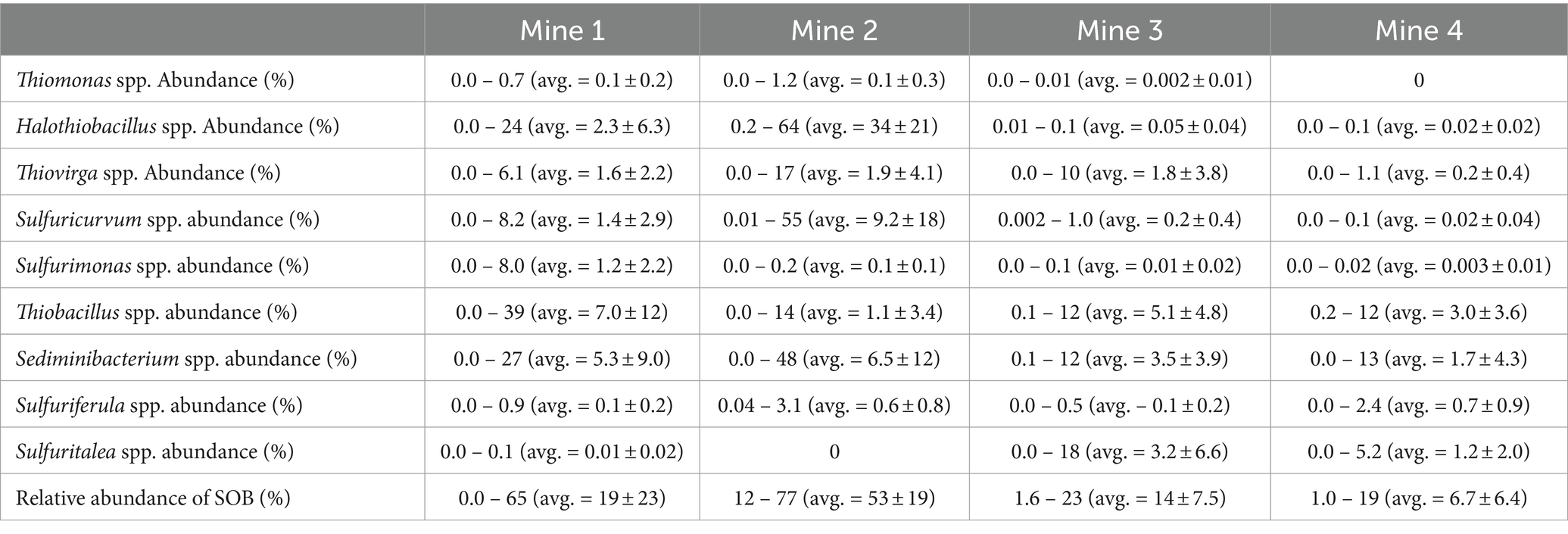
Table 2 . Average (± standard deviation), minimum and maximum 16S rRNA percent relative abundances across sulfur oxidizing bacteria genera data for Mine 1, Mine 2, Mine 3, and Mine 4 tailings impoundment waters (2016 – 2019).
To identify the potential sulfur metabolizing pathways encoded by these SOB genera, 116 metagenomes were constructed from 38 samples collected at these four mines between 2016 and 2018 ( Supplementary Table 2 ). Genomes for Sulfurimonas and Sulfuritalea could not be reconstructed and thus interpretations for these two genera relied on literature reports. Three major pathways including sox , r dsr , and S 4 I were examined, as well as additional sulfur oxidation genes not specific to those three pathways ( Figure 1 ), based on the foundational understanding outlined by Watanabe et al. (2019) and expanded by Whaley-Martin et al. (2023) . Genes encoding the sox pathway, either complete (c sox ; soxXYZABCD ) or incomplete (i sox ; soxXYZAB and lacking soxCD ) were most common – occurring in eight of the nine genera. Thiomonas , Halothiobacillus, and Thiovirga genomes encoded the c sox pathway (resulting in generation of SO 4 2- ; Figure 1 ) which is consistent with published reports for Thiomonas and Halothiobacillus ( Veith et al., 2012 ; Lin et al., 2015 ). Outside of other published works from the mines included in this study, there are very limited data available in the literature for Thiovirga spp., Miettinen et al. (2021) identified possible Thiovirga spp. in zinc and copper ore processing facilities in Portugal that lacked only the soxC subunit, indicating there may be variability within the Thiovirga genus regarding the ability for complete oxidation to SO 4 2- via the sox pathway, unlike the findings presented here ( Figure 1 ). Sulfurimonas spp. have been reported to often possess the soxCD genes (i.e., c sox ) ( Lahme et al., 2020 ; Wang et al., 2021 , 2023 ) however, this genus may lack soxAB genes instead, which would not allow for complete oxidation via the sox pathway ( Lahme et al., 2020 ; Wang et al., 2021 ). At least one strain of Sulfurimonas (Strain NW10 T ) has been reported to possess genes for the c sox pathway but used the i sox pathway instead ( Wang et al., 2021 ).
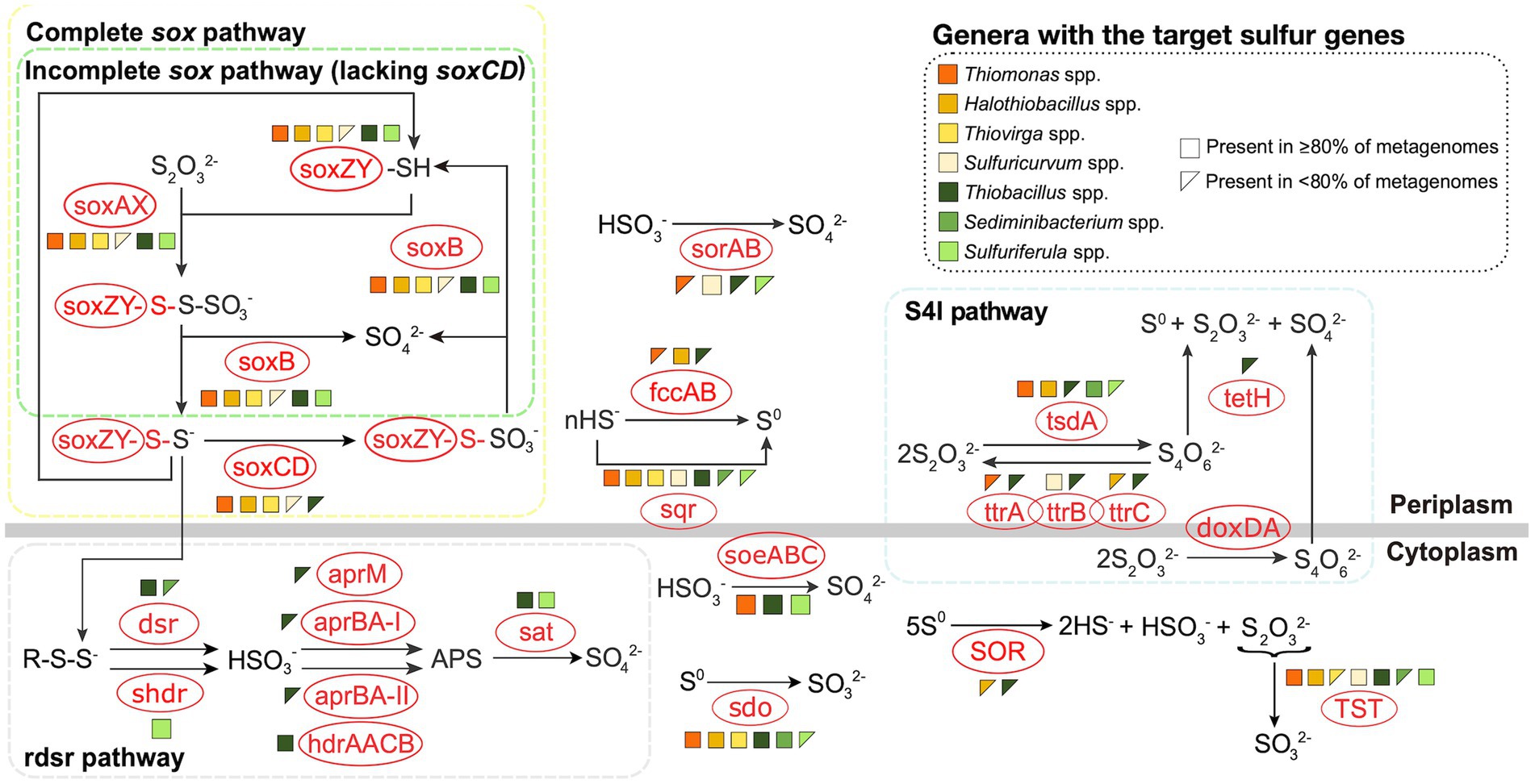
Figure 1 . Metabolic potentials and pathways for sulfur oxidizing bacteria genera from the four TIs investigated here. Figure was adapted from Whaley-Martin et al. (2023) and based on Watanabe et al. (2019) . Genomes were reconstructed from TI samples collected from the four target mines between 2016 and 2018. Due to low abundance of some organisms, variable quantities of genomes were used including 3 Thiomonas , 31 Halothiobacillus , 3 Thiovirga , 31 Thiobacillus , 30 Sediminibacterium , 4 Sulfuricurvum , and 14 Sulfuriferula .
Sediminibacterium was the only detected SOB in this study that did not encode any sox genes ( Figure 1 ). Two genera were identified as possessing the i sox pathway; Sulfuriferula and Thiobacillus ( Figure 1 ). Watanabe et al. (2019) reported Sulfuritalea hydrogenivorans sk43H, which at present is the only published pure culture strain, as possessing soxAX , soxYZ , and soxB but lacking soxCD, thus indicating the presence of the i sox pathway. Three of the reconstructed Sulfuricurvum spp. genomes encoded c sox, while one encoded no sox genes ( Figure 1 ), with the latter potentially due to the low quality of the genome. This finding of c sox encoding Sulfuricurvum from mining TI samples described here, and also reported in Whaley-Martin et al. (2023) , diverges from the current literature which typically reports Sulfuricurvum spp. found in the natural environment (terrestrial aquifer, geothermal springs) as possessing the i sox pathway (lacking soxCD ; Handley et al., 2014 ; Meziti et al., 2021 ).
A S 4 I pathway gene for the first reaction generating S 4 O 6 2- , was the second most abundant identified across the seven genera with reconstructed genomes ( Figure 1 ). Two distinct catalysts, tsdA and doxDA, are responsible for the conversion of S 2 O 3 2- to S 4 O 6 2- ( Nguyen et al., 2022 ). However, doxDA was absent in the reconstructed genomes, while tsdA was present in the genomes of five of the seven genera, including >80% of Thiomonas , Halothiobacillus , and Sediminibacterium genomes and < 80% of Thiobacillus and Sulfuriferula genomes ( Figure 1 ). Generation of tetrathionate from tsdA activity can be subsequently disproportionated via tetH to form S 0 , S 2 O 3 2- , and SO 4 2- , which can then potentially feed both the incomplete/complete sox pathway and/or S 0 storage pathways (including sdo , SOR, and r dsr ; Friedrich et al., 2005 ; Frigaard and Dahl, 2008 ; Watanabe et al., 2019 ). Though tsdA was found in five genomes, tetH, which would be required for the completion of the S 4 I pathway, was only found in five of the thirty-one Thiobacillus genomes (<80%; Figure 1 ). This may indicate the potential for genera with only tsdA (such as Thiomonas , Halothiobacillus , Sediminibacterium and Sulfuriferula ) to couple with tetH encoding Thiobacillus to complete the S 4 I pathway. Limited tetH detection may also reflect current limitations in tetH reference material. TtrABC can catalyze the reduction of S 4 O 6 2- to produce S 2 O 3 2- though its components ( ttrA, ttrB , and ttrC ) were only variably present across Thiobacillus genomes ( Figure 1 ). Thiomonas spp., Sulfuricurvum spp., and Halothiobacillus spp. were found to possess ttrA, ttrB , and ttrC respectively, but there is no available research indicating these subunits are active in sulfur reduction individually. Watanabe et al. (2019) reported that doxDA , tsdA , and tetH genes were absent in Sulfuritalea hydrogenivorans sk43H indicating this Sulfuritalea species did not encode the S 4 I pathway. Currently, there is a lack of specific data regarding the presence or activity of the S 4 I pathway (and associated genes tsdA , doxDA , and tetH ) within Sulfurimonas . Consequently, it remains uncertain in results here and in available literature, whether Sulfurimonas possess the genomic capacity to support the S 4 I pathway.
The genes for the complete rdsr pathway were detected in 29 of the 31 Thiobacillus genomes ( Figure 1 ). Sulfuriferula possessed the potential to express shdr (SO 3 2- production) and sat (SO 4 2- production through APS) but lacked the intermediate aprM/aprBA- I or aprBA-II/hdrAACB genes that mediate the reaction produce APS ( Figure 1 ), aligning with results presented in Watanabe et al. (2019) for Sulfuriferula sp. AH1 and Sulfuriferula thiophila mst6. These missing steps could be compensated for by the cytoplasmically oriented soeABC that was present in all Sulfuriferula genomes, which generates SO 4 2- directly from SO 3 2- ( Koch and Dahl, 2018 ; Figure 1 ). Alternatively, SO 3 2- can be transported to the periplasm via TauE -like transporter where it can spontaneously react with hydrogen sulfide if present ( Koch and Dahl, 2018 ). The lack of rdsr and shdr pathway genes in the SOB identified in this study, may reflect the relatively high dissolved oxygen concentrations across these water samples (averaging between 36.2 – 82.6 % saturation across the four mines; Table 1 ) which do not favour establishment of anaerobic and/or microaerophilic SOB which typically harbour the rdsr pathway ( Klatt and Polerecky, 2015 ). Isolated genomes of Thiobacillus contained aprM , aprBA-I , aprBA-II , and/or hdrAACB, which would allow them to further oxidize SO 3 2- to APS ( Figure 1 ). Watanabe et al. (2019) reported the presence of partial (lacking aprM and aprBA-I ) rdsr pathway genes (including dsrAB , dsrEFH , dsrC, dsrMKJOP, and sat ) in the model species Sulfuritalea hydrogenivorans sk43H . Furthermore, evidence reported by Purcell et al. (2014) indicates Sulfuritalea hydrogenivorans sk43H can carry out the rdsr pathway within Antarctic lake sediments. Sulfuritalea hydrogenivorans sk43H also hosts a partial s hdr pathway including aprBA-II and hdrAACB ( Watanabe et al., 2019 ). Second only to the Thiobacillus genomes presented here, Sulfuritalea hydrogenivorans sk43H hosts the most diverse set of r dsr pathway genes of the nine SOB genera identified. While there are several published Sulfurimonas genomes, detailed insights into r dsr pathway occurrence remains limited. This may be due to the absence of the rdsr pathway within the Sulfurimonas genus as Wang et al. (2023) reports Sulfurimonas sp. ST-27 lacks dsrAB , dsrE , dsrCD , dsrMKJOP, and aprBA, but does contain sat . The rdsr and shdr pathways may play an important role in bacteria harbouring the i sox pathway by facilitating the conversion of sulfane sulfur, produced as an intermediate, into SO 4 2- ( Xin et al., 2023 ). SOB that encode the i sox pathway without the r dsr or s hdr pathways, may produce and accumulate sulfane sulfur as a by-product of S 2 O 3 2- oxidation, though the mechanism for reducing oxidative stress from the accumulation of sulfane sulfur is not well delineated and may result in the accumulation of volatile H 2 S ( Xin et al., 2023 ). Additionally, sulfane sulfur has been found to exhibit toxicity in both bacteria and fungi, including when produced through the i sox pathway in Cupriavidus pinatubonensis ( Sato et al., 2011 ; Xin et al., 2023 ). Of the four genera in this study which contain the i sox pathway, only Sulfuritalea and Thiobacillus possess the ability to detoxify sulfane sulfur using either the r dsr or s hdr pathway.
Additional cytoplasmic sulfur oxidative genes investigated include TST (S 2 O 3 2- to SO 3 2- ) and soeABC (SO 3 2- to SO 4 2- ; Figure 1 ). TST was broadly present across the SOB occurring in >80% of the Thiomonas spp., Halothiobacillus spp., Thiobacillus spp., Sulfuricurvum spp., and Sulfuriferula spp. metagenomes and in <80% of the Thiovirga spp. and Sediminibacterium spp. metagenomes ( Figure 1 ). To date, no information is available regarding the presence or absence of TST in Sulfurimonas or Sulfuritalea genomes. Wang et al. (2019) suggests TST may use S 2 O 3 2- produced by SOR to further oxidize it to sulfur and SO 3 2- . SoeABC transforms SO 3 2- to SO 4 2- in the cytoplasm and may even contribute to SO 3 2- oxidation as part of the r dsr pathway ( Dahl et al., 2013 ; Whaley-Martin et al., 2023 ). Greater than 80% of the Thiomonas, Thiobacillus and Sulfuriferula genomes contained soeABC ( Figure 1 ). SoeABC has also been identified in Sulfuritalea hydrogenivorans sk43H ( Watanabe et al., 2014 ) but no information on soeABC ’s occurrence in Sulfurimonas is currently available. Additional periplasmic sulfur oxidation genes identified in this study include sqr , fccAB, and sorAB ( Figure 1 ). Both sqr and fccAB are implicated in catalysis of hydrogen sulfide to S 0 which can form S 0 globules and be further oxidized to SO 3 2- via the r dsr pathway ( Nosalova et al., 2023 ). Sqr was widely present appearing in >80% of the genomes identified in all genera apart from Sediminibacterium and Sulfuriferula metagenomes where they were present in <80% of the metagenomes ( Figure 1 ). Sulfuritalea hydrogenivorans sk43H and various Sulfurimonas species were found to host the s qr gene ( Watanabe et al., 2019 ; Wang et al., 2021 , 2023 ). Among the reconstructed genomes, >80% of the Halothiobacillus genomes harbored the fccAB gene cluster while the fccAB cluster was identified in <80% of the Thiomonas and Thiobacillus genomes ( Figure 1 ). Sulfuritalea hydrogenivorans sk43H has been reported to contain fccAB ( Watanabe et al., 2019 ) and currently no Sulfurimonas species have been reported to contain fccAB . SorAB , which catalyzes the conversion of SO 3 2- to SO 4 2- in the periplasm, was found in >80% of the Sulfuricurvum genomes and < 80% of the Thiomonas, Thiobacillus , and Sulfuriferula genomes ( Figure 1 ). SorAB has also been reported in Sulfuritalea hydrogenivorans sk43H ( Watanabe et al., 2019 ) as well as eight of the eleven Sulfurimonas species examined by Wang et al. (2021) . The four most abundant genera in these four TI collectively possess the capacity for sulfur oxidation via all three universal pathways, c sox ( Halothiobacillus , Sulfuricurvum ), i sox + rdsr ( Thiobacillus ), and S 4 I ( Halothiobacillus, Sediminibacterium , Thiobacillus ) suggesting adaptation of these TI SOB communities to occupy all potential sulfur oxidizing niches that occur in these highly physicochemically and geochemically dynamic TI systems.
3.1.1 SOB functional classification
Under acid mine drainage conditions (acidic, metal rich), Kuang et al. (2016) identified metabolic function as a better predictor of microbial community structure and function than taxonomy. Similarly, here, patterns in metagenomic data were used to classify these SOB genera into c sox dominant and non-c sox dominant SOB genera groupings. C sox dominant SOB genera identified here included Halothiobacillus spp., Thiovirga spp., Thiomonas spp., and Sulfuricurvum spp. based on the presence and high abundance of the c sox pathway ( Figure 1 ). Non-c sox dominant SOB genera were characterized by i sox gene pathway presence and increased abundance of genes associated with alternative pathways such as r dsr ( dsrABCEFH , aprAB , sat ; Whaley-Martin et al., 2023 ) and/or S 4 I ( tsdA, tetH ; Ghosh and Dam, 2009 ; Wang et al., 2019 ; Whaley-Martin et al., 2023 ) ( Figure 1 ). Thiobacillus spp., Sediminibacterium spp., Sulfuriferula spp., Sulfuritalea spp., and Sulfurimonas spp. were categorized as non-c sox dominant SOB genera ( Figure 1 ). Importantly, reactions catalyzed by the c sox pathway favour the complete oxidation of SOI to SO 4 2- , resulting in increased acidity and SO 4 2- production, while more energy efficient pathways (e.g., r dsr ; Klatt and Polerecky, 2015 ), favoured by non-c sox dominant SOB commonly generate free SOI and in some cases may consume H + ( Dam et al., 2007 ; Klatt and Polerecky, 2015 ; Hutt et al., 2017 ). Therefore, presence or absence of c sox vs. non-c sox dominant pathways may reflect, and in turn, result in physicochemically and geochemically distinct waters. Mine waters with non-c sox dominant SOB (i.e., Thiobacillus , which can generate free SOI) may be at greater risk of offsite acidification due to the ability of many SOI to pass through current treatment to receiving environments where their subsequent oxidation could release acidity.
3.1.2 Spatial and temporal trends in SOB community composition and function
The abundance of the nine identified SOB genera differed spatially and temporally for individual mines as well as across the four mines ( Figure 2 ). The two oldest TIs, Mine 2 and Mine 1, had the highest SOB abundances ( Figure 2 ). Mine 2 TI had the largest total abundance of SOB genera across all samples (54 ± 19%) and was the only mine TI where c sox dominant SOB genera (predominantly Halothiobacillus spp.) had a higher average abundance than non-c sox dominant SOB genera (predominantly Sediminibacterium spp.; Figure 2 , Table 2 ). With more than 50% of the community on average consisting of SOB, Mine 2 TI had the lowest non-SOB genera abundance (38 ± 17%) and lowest unknown genera abundance (12 ± 9.4%; Figure 2 ). Based on evidence from this study and a previous study ( Whaley-Martin et al., 2023 ), the Mine 2 community had the potential to express the c sox pathway (via Halothiobacillus spp., Thiovirga spp., Thiomonas spp. or Sulfuricurvum spp.), i sox pathway (via Thiobacillus spp. or Sulfuriferula spp.), r dsr pathway (via Thiobacillus spp.), and/or the S 4 I pathway (via Thiobacillus spp.). The average c sox dominant SOB abundance was significantly higher in Mine 2 ( p < 0.001) compared to the other three mines, while the abundance of non-c sox dominant SOB were not significantly different across the four mines. Averaging a total SOB abundance 2.7 times lower than Mine 2, Mine 1 TI had the second highest total SOB abundance (20 ± 24%) ( Figure 2 , Table 2 ). SOB genera present at Mine 1 could express the c sox pathway (via Halothiobacillus spp., Thiovirga spp., or Sulfuricurvum spp.), i sox pathway (via Thiobacillus spp. or Sulfurimonas spp.), r dsr pathway (via Thiobacillus spp.), and/or the S 4 I pathway (via Thiobacillus spp.).
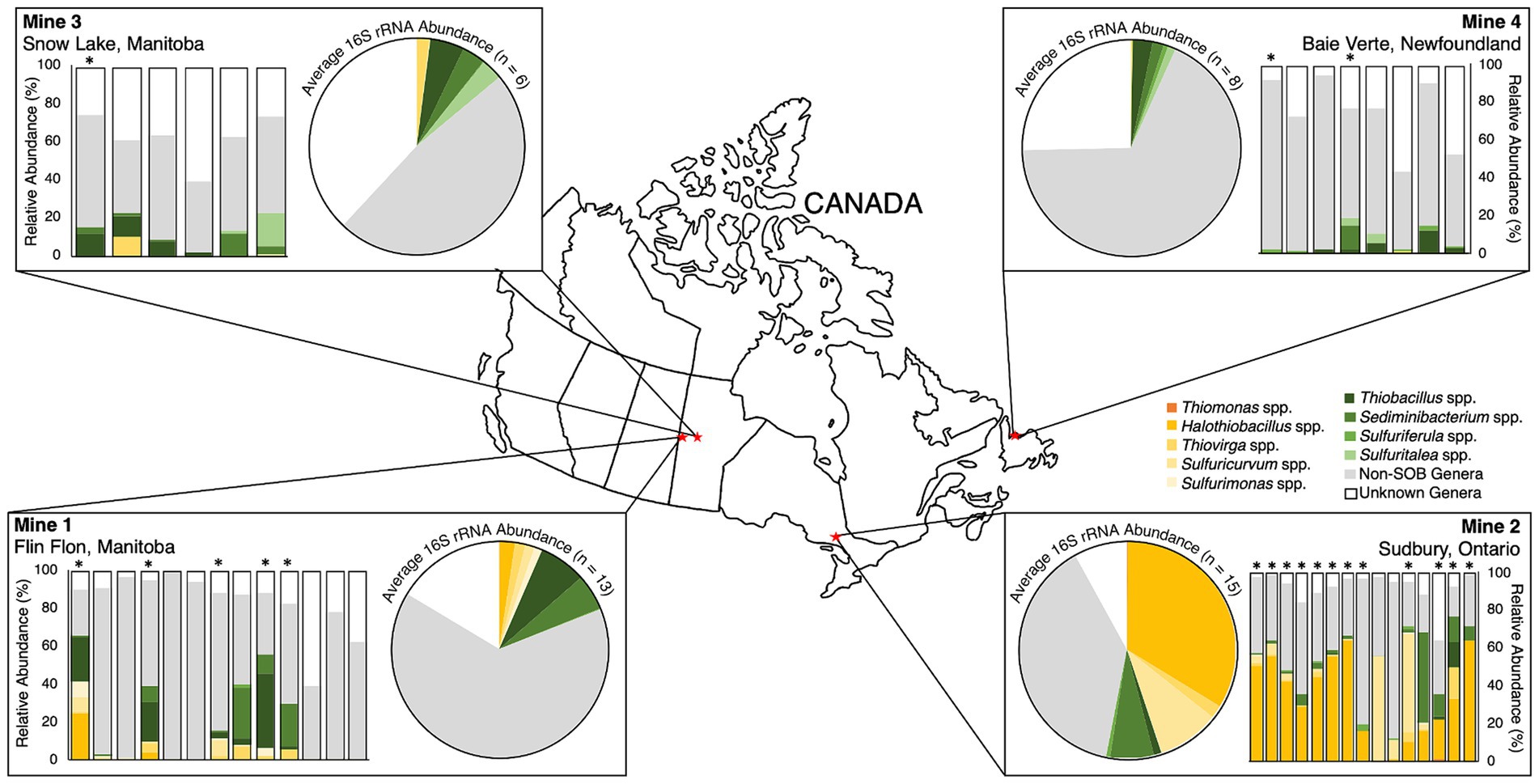
Figure 2 . 16S rRNA relative abundance (%) of top nine sulfur oxidizing bacteria genera from 2016 – 2019, non-SOB genera and unknown genera for each of the four mines mapped to their sample locations across Canada. Individual samples are shown as bar graphs and mine average abundances are shown as pie graphs. “Non-SOB genera” are all other identified sequences and “unknown genera” are identified sequences not matched to genera in the Silva Database v138.1. Asterisks (*) denote samples with metagenome data included in this study.
Mine 3’s TI waters averaged 14 ± 7.5% total SOB abundance ( Table 2 ), which was approximately 4 times lower than Mine 2 and 1.4 times lower than Mine 1. Mine 3 TI did, however, have the highest abundance of unknown genera (38 ± 12%) and the second highest abundance of identified non-SOB genera (48 ± 8.2%; Figure 2 ). The majority of TI SOB present in Mine 3 TI waters were identified as non-c sox dominant SOB and averaged 12 ± 6.2% primarily consisting of Thiobacillus spp., Sediminibacterium spp. and Sulfuritalea spp. ( Figure 2 , Table 2 ). The average abundance of c sox dominant SOB genera at Mine 3 was 2.0 ± 3.7% and was primarily Thiovirga spp. ( Figure 2 , Table 2 ). 16S rRNA abundance, metagenome data and present literature indicate that SOB genera from Mine 3 could express the c sox pathway (via Thiovirga spp.) and/or the i sox (via Thiobacillus spp. or Sulfuritalea spp.), r dsr (via Thiobacillus spp.), and S 4 I pathway (via Thiobacillus spp.).
The lowest total SOB abundance was found in the Mine 4 TI samples, with an average abundance of 7.1 ± 6.3 and > 97% of the SOB present represented by non-c sox dominant SOB genera ( Table 2 ). Non-c sox dominant SOB genera averaged 6.5 ± 6.6% of the overall community abundance, while c sox dominant SOB abundance was the lowest of the four mines averaging 0.3 ± 0.4%, with only Thiovirga spp. ever reaching above 1% of the overall community abundance at Mine 4 ( Table 2 ). Mine 4 had the second highest contribution of unknown genera representing 27 ± 17% of the microbial community ( Figure 2 ). Mine 4 had the most limited S oxidation potential with no major SOB c sox abundance ( Table 2 ). Thiobacillus spp. present at Mine 4, contained tsdA enabling part 1 of the S 4 I pathway (S 4 O 6 2- formation), but did not contain tetH associated with the 2 nd part of the S 4 I pathway (S 4 O 6 2- disproportionation) and did contain genes for the i sox and r dsr pathway.
3.2 TI wastewater sulfur geochemistry
Mean [total S 0.45μm ] and [SO 4 2- ] across the four mines display a pattern of increasing concentration with age, though this relationship is not proportional. Notably, Mine 1 and Mine 2 are most similar in age and Mine 2 and Mine 3 are most similar in concentrations ( Table 1 , Figure 3 ). However, [total S 0.45μm ] exhibited substantial variability across the four mines – ranging from 0.3 mM (Mine 4) to 16.8 mM (Mine 1) ( Table 1 ). ANOVA and post-hoc Tukey pairwise comparison tests revealed a significantly higher average total S 0.45μm concentration at Mine 1 (14 ± 1.6 mM) compared to Mine 2 (8.9 ± 0.7), Mine 3 (7.9 ± 1.0), and Mine 4 (1.9 ± 0.8 mM) ( Table 1 ; p < 0.001). [SO 4 2- ] displayed a similar trend where Mine 1 exhibited significantly higher concentrations than Mine 2 ( p < 0.05), Mine 3 ( p < 0.001), and Mine 4 ( p < 0.001) ( Table 1 , Figure 3 ). Elevated sulfur concentrations in Mine 1 and Mine 2 TIs can be attributed to their extensive use since the 1920s. Mine 3, while younger than Mine 1 and Mine 2, contains a comparatively higher [total S 0.45μm ], reflecting tailings additions from multiple mining operations and a shallow water cover depth. Mine 4, the smallest and youngest TI, has accumulated the lowest volume of tailings, resulting in the lowest [total S 0.45μm ].
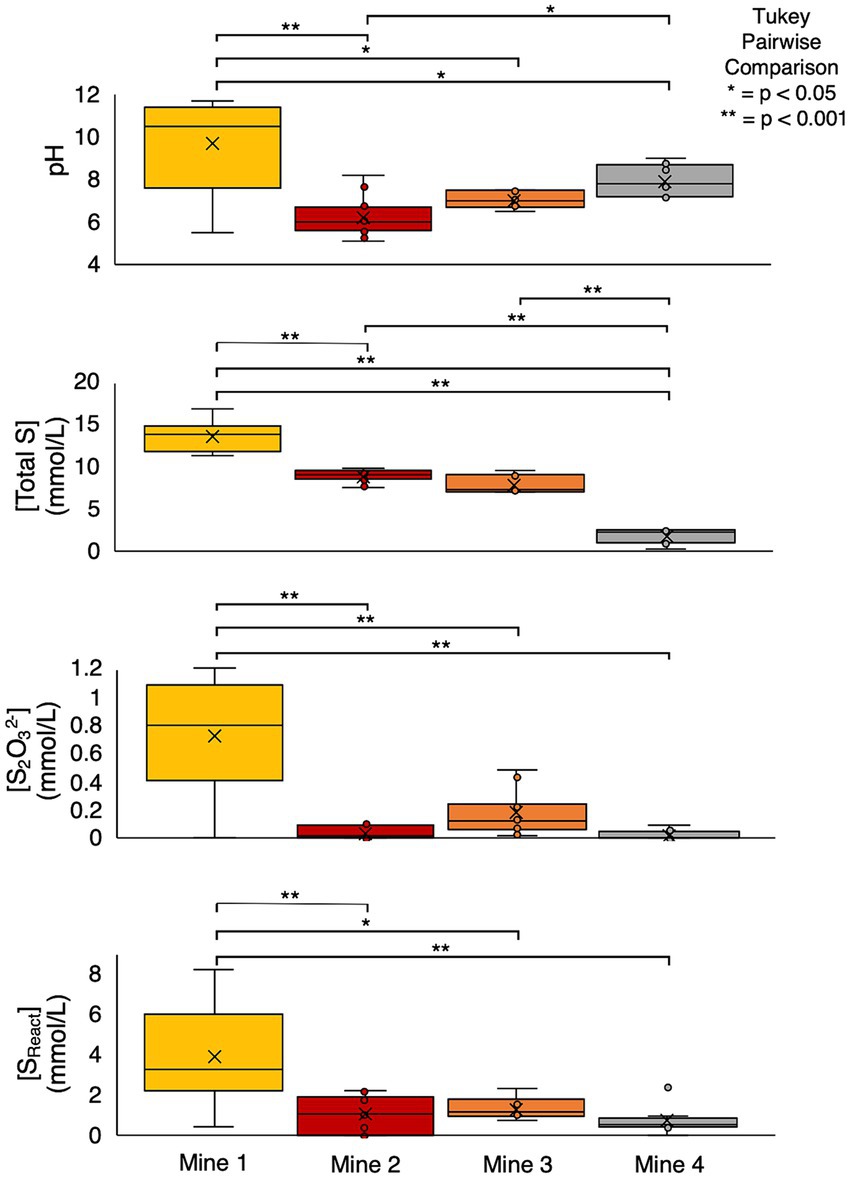
Figure 3 . Box and whisker plots and statistical analysis (ANOVA and post-hoc tukey pairwise statistical comparison) of cross mine pH, total S (mmol/L), S 2 O 3 2- (mmol/L), and S React (mmol/L). Box limits represent the first and third quartile of each dataset, with a black line indicating the median value and an “x” denoting the mean.
Though not often reported, [total S] from various environments varies widely from ~10 μM to 800 μM observed in freshwater lakes ( Vincent et al., 2021 ) to ~28 mM in sea water ( Canfield and Farquhar, 2009 ; Vincent et al., 2021 ) with SO 4 2- typically being the largest contributor to the overall sulfur balance ( Canfield and Farquhar, 2009 ). Reported [SO 4 2- ] from other mining environments studies range from 3.6 mM to >250 mM ( Foucher et al., 2001 ; Silva et al., 2012 ; Kinnunen et al., 2018 ; Camacho et al., 2020b ). [Total S] (0.3 – 16.8 mM; Table 1 ) in this study are typically higher than the reported freshwater <1 μM value, while on the lower end of reported values for mining contexts ( Foucher et al., 2001 ; Kinnunen et al., 2018 ).
Reactive sulfur concentrations ([S React ] calculated as [Total S] – [SO 4 2- ]; Whaley-Martin et al., 2020 ), representing all sulfur atoms capable of oxidation, were determined for each TI sample. ANOVA and post-hoc Tukey pairwise comparison tests revealed that Mine 1 had a significantly higher average S React compared to Mine 2 ( p < 0.001), Mine 3 ( p < 0.05) and Mine 4 ( p < 0.001) ( Figure 3 ). Considering the potential 10-fold variation in both [total S 0.45μm ] and [S React ] between samples, %S React ([S React ]/[Total S 0.45μm ] x 100) provides a useful metric to reflect the proportion of S React within each system’s individual total S pool. %S React ranged from 0 to 92.3% across the four mines, with the highest average %S React occurring at Mine 1 (28 ± 16.2%) followed by Mine 4 (27 ± 13%), Mine 3 (17 ± 4.4%), and Mine 2 (12 ± 9.5%). S React may include various sulfur species such as S 2 O 3 2- and SO 3 2- (quantified in this study), as well as additional unresolved sulfur species including but not limited to S 4 O 6 2- , S 2 O 4 2- , and S 0 which were not quantified here but could potentially contribute to or support microbial sulfur cycling ( Bak and Pfennig, 1987 ; Kelly et al., 1997 ). [SO 3 2- ] were generally low (often at or near the detection limit) with the highest concentrations found at Mine 1 (0.03 ± 0.05 mM; Table 1 ). S 2 O 3 2- however, was detectable across all sites with a significantly higher ( p < 0.001) average concentration at Mine 1 compared to the other three mines ( Figure 3 ). [S 2 O 3 2- ] was the largest measured S React contributor, constituting 0 – 100% (calculated %S 2 O 3 2- = [S 2 O 3 2- ]/[S React ] x 100) of the S React pool at each mine. While Mine 4 had the largest average %S React , it had the smallest average %S 2 O 3 2- (5.3 ± 8.2%) and therefore the largest proportion of unresolved sulfur species. On average, [S 2 O 3 2- ] comprised approximately one-fifth of both Mine 1 (22 ± 16%) and Mine 2’s (19 ± 27%) [S React ] pool.
3.3 TI wastewater physicochemistry
42 TI water cap samples were collected from the four mines between 2016 and 2019 during open water periods (early spring to late fall; Supplementary Figure S1 ) exhibited variable pH and DO values which are known to be important influencers of microbial ecological niches and associated sulfur oxidation pathways ( Chen et al., 2013 ; Whaley-Martin et al., 2023 ). High temporal variability in DO (% saturation) and pH occurred for each mine as well as across mines, reflecting morphometric differences between TI facilities (particularly depth; Table 1 ) and the dynamic nature of actively managed TIs ( Figure 3 ). Oxygen profiles from Mine 1, Mine 2, and Mine 3 demonstrated a steep oxygen gradient within TI water caps (Mine 4 profile data unavailable), ranging from <1 to >100% saturation ( Table 1 ) across the four mines. Cross mine comparison revealed Mine 4 had significantly higher %DO compared to Mine 1 and Mine 2 (ANOVA and a post-hoc Tukey pairwise comparison test, p < 0.05; Figure 3 ) and no statistically significant differences among the remaining mines %DO.
Across the four mines, pH ranged from 5.1 to 11.8 ( Table 1 ; Figure 3 ). Lime (Ca(OH) 2 ) and alkaline tailings additions resulted in the highest average pH at Mine 1 (9.7 ± 2.0) and significantly higher pH values than Mine 2 ( p < 0.001), Mine 3 ( p < 0.05), and Mine 4 ( p < 0.05) ( Figure 3 ). Mine 2 TI waters had the lowest average pH (6.3 ± 0.9) with pH regularly falling below pH 6.5 (~64% of datapoints) and an observed decrease in pH from late spring to early fall. Mine 3’s TI water cap had the narrowest range of pH values (6.6 – 7.6, avg. = 7.1) with no observed seasonal fluctuations. Mine 4’s average pH was 8.0 ± 0.7 (7.2 – 9.0), with 75% of samples below pH 8.5, which was significantly higher than Mine 2 ( p < 0.05) and lower than Mine 1 ( p < 0.05; Figure 3 ). All four TIs exhibit dimictic lake mixing patterns, resulting in more homogenous dissolved oxygen and pH profiles during turnover events (spring and fall).
The acidity to SO 4 2- ratio ([H + ]/[SO 4 2- ]) can be used as a proxy for discerning direct sulfur oxidation from disproportionation ( Bernier and Warren, 2007 ; Whaley-Martin et al., 2023 ). Higher [H + ]/[SO 4 2- ] values observed at lower pH values indicate greater dominance of the c sox pathway, indicating more direct and complete oxidation to SO 4 2- (higher acidity generation, minimal SOI generation; Supplementary Figure S3 ). Lower [H + ]/[SO 4 2- ] values observed at pH values >7, are consistent with more regeneration of SOI via disproportionation or partial oxidation attributed to i sox , r dsr , and S 4 I pathways activity, resulting in lower net acidity generation ( Supplementary Figure S3 ). While these ratio values may be underestimates at pH values >7, where buffering capacity is likely present, the observed pH dependent [H + ]/[SO 4 2- ] trend is consistent with both S 2 O 3 2- and SOB results. [S 2 O 3 2- ] was positively correlated to pH ( p < 0.0001, Pearson’s r = 0.80; Supplementary Figure S4A ), while total TI SOB abundance was negatively correlated with pH ( p < 0.01, Pearson’s r = −0.64; Supplementary Figure S4B ). Interestingly, average mining and anthropogenic (M&A) literature and environmental literature values were consistent with the pattern observed here ( Supplementary Figure S4B ).
3.4 pH effect on SOB community structure
Further examination of the relationship between SOB and pH for these four mine TIs, highlighted a pH dependent pattern in the occurrence of c sox and non-c sox dominant SOB. While clear relationships between pH and total SOB abundance, [S 2 O 3 2- ], and [H + ]/[SO 4 2- ] ratios were observed, no discernible correlation was identified between dissolved oxygen and any of these parameters. Peak abundances of non-c sox dominant SOB were observed at circumneutral pH (pH ~6 to ~8.5; Figure 4Ai ) while the highest abundances of c sox dominant SOB were observed below pH ~6.5 ( Figure 4Aii ). Samples above pH ~8.5 had low abundances of both c sox and non-c sox dominant SOB ( Figures 4Ai,ii ). Current literature indicates at least two genera of SOB ( Thioalkalimicrobium and Thioalkalivibrio ) are capable of growth under alkaline conditions ( Sorokin et al., 2001 ) though neither were observed in this study’s TI samples. Lower pH value (pH < ~6.5) TI waters had the highest abundances of c sox dominant SOB, lower [S 2 O 3 2- ] and higher [H + ]/[SO 4 2- ] ratios; collectively consistent with direct oxidation via the c sox pathway ( Figure 4Aii ). At more circumneutral pH values (pH ~6 to ~8.5), where non-c sox dominant SOB dominated, higher [S 2 O 3 2- ] and low [H + ]/[SO 4 2- ] values were also observed, indicating distinct pH dependent SOB community structure and S pathway(s) ( Figures 4Ai,ii ).
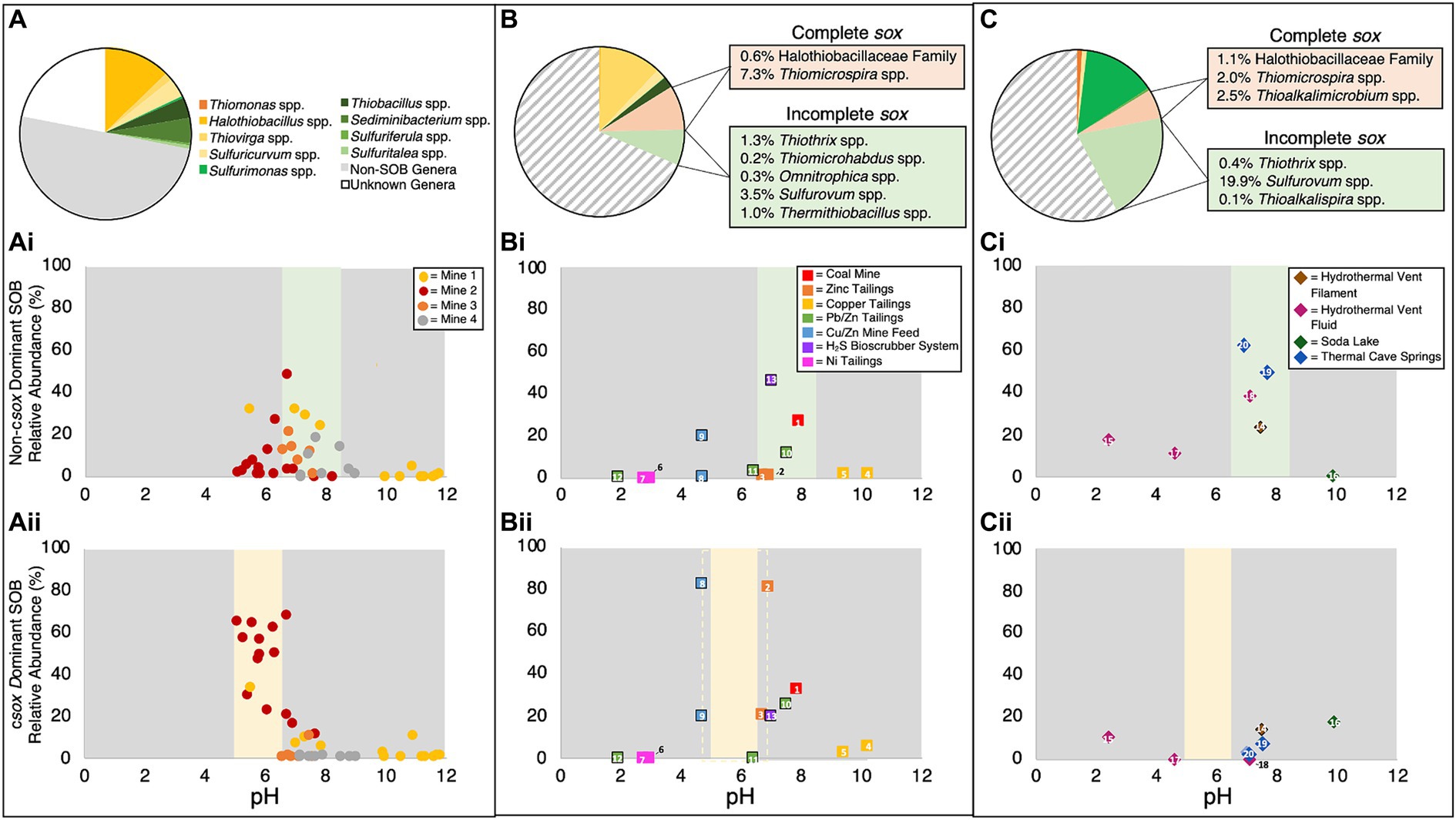
Figure 4 . Genus level identification of (A–C) c sox dominant SOB relative abundances, (Ai, Bi, Ci) non-c sox dominant SOB relative abundances and (Aii, Bii, Cii) average 16S rRNA SOB communities identified in (A, Ai, Aii) tailings impoundment water samples from this study, (B, Bi, Bii) other mining and anthropogenic sample data from the literature and (C, Ci, Cii) environmental sample data obtained from the literature. “Non-SOB genera” are all other identified sequences in the samples and “unknown genera” are identified sequences not matched to genera in the Silva Database v138.1 (Data included in Bi, Bii, Ci , and Cii was collected from the following papers and can be identified using the superscript number: 1 Kadnikov et al., 2019 , 2-5 Miettinen et al., 2021 , 6-7 Auld et al., 2017 , 8-9 Lopes et al., 2020 , 10-12 Chen et al., 2013 , 13 Haosagul et al., 2020 , 14 Patwardhan et al., 2018 , 15 Arce-Rodríguez et al., 2019 , 16 Vavourakis et al., 2019 , 17-18 Meier et al., 2017 , 19-20 Reigstad et al., 2011 ). Black outlines indicate non-water samples (e.g., soil, rock, tailings, biofilm, etc.).
3.4.1 Comparison to broader environments
The exploration of the pH – c sox dominant/non-c sox dominant SOB relationship extended across diverse contexts by integrating literature data (pH and SOB abundance) from broader mining environments, an industrial H 2 S bioscrubber, and a variety of natural environmental sites (e.g., hydrothermal vents, soda lakes, and thermal cave springs) ( Reigstad et al., 2011 ; Meier et al., 2017 ; Patwardhan et al., 2018 ; Arce-Rodríguez et al., 2019 ; Vavourakis et al., 2019 ) (see Supplementary Table S1 ). Genus level SOB abundances were categorized into c sox dominant SOB and non-c sox dominant SOB. Literature identified SOB (not found in this study’s TI samples) were classified as c sox dominant and non-c sox dominant SOB using available genetic data and consensus of the current literature based on the reported presence of a complete or incomplete sox pathway. Additional SOB genera included three c sox dominant clades Thiomicrospira spp., Thioalkalimicrobium spp., and Halothiobacilleaceae Family and seven non-c sox dominant clades ( Thiothrix spp., Thiomicrohabdus spp., Omnitrophica spp., Sulfurovum spp., Thermithiobacillus spp., Sulfobacillus spp., and Thioalkalispira spp.). Though class and family level abundances have been published for TI water cap samples, genus level data were not widely available in the literature. Data from various alternative sample locations (e.g., water from mill feed, solid tailings pore water), including both solid and water samples, were used. This approach aimed to broaden the range of geochemical and physicochemical characteristic investigated.
A brief summary and comparison of the relevant data for the overall groups of samples are provided in Supplementary Table S1 as more complete descriptions for individual sites can be found in their respective publications. The collective M&A literature data ( n = 13) covered a pH range from 1.9 to 10.2 ( Supplementary Table S1 ), the environmental samples ( n = 7) ranged from pH 2.4 to 9.9 ( Supplementary Table S1 ), while this study’s TI samples ranged from pH 5.1 to 11.8 ( Table 1 ). Average total SOB abundance of this study’s TI samples (29 ± 26%) and M&A literature samples (31 ± 31%) were similar, while the average total SOB abundance for the environmental literature samples (42 ± 26%) was >10% higher ( Figures 4A – C ). Additional SOB genera (outside of the nine identified for TI in this study) represented ~15% (or ~ 50% of the total SOB) of the M&A literature and ~ 26% (or ~ 62% of the total SOB) of the environmental literature samples ( Figures 4B , C ). Both the M&A and environmental literature SOB communities diverged by at least 50% from those characterized here for base metal TI wastewaters, indicating notable disparities in microbial community composition suggesting SOB genera specific ecological niches.
On average, this study’s samples had ~64% of the total SOB abundance consisting of c sox dominant SOB, while ~36% were classified as non-c sox dominant ( Figure 4A ). The M&A literature samples averaged slightly higher c sox dominant SOB contribution with ~72% of the total SOB abundance being attributed to c sox dominant SOB ( Figure 4B ). Conversely, the environmental literature samples averaged a much lower contribution of c sox dominant SOB, contributing only ~18% of the total SOB abundance ( Figure 4C ). Thiovirga spp. and Thiomicrospira spp., both classified as c sox dominant SOB, were the two most abundant SOB in the M&A literature samples, while the two most abundant SOB in the environmental samples were both classified as non-c sox dominant SOB ( Sulfurimonas spp. and Sulfurovum spp.) ( Figures 4B , C ; Supplementary Table S1 ). In contrast, the two most abundant SOB from this study’s TI waters were Halothiobacillus spp. (c sox dominant) and Sediminibacterium spp. (non-c sox dominant). Thiomicrohabdus spp., Omnitrophica spp., and Thermithiobacillus spp. were unique to M&A samples with Thiomicrohabdus spp. and Omnitrophica spp. only being found in a single sample ( Kadnikov et al., 2019 ) and Thermithiobacillus spp. identified in two samples from the same mine ( Lopes et al., 2020 ). Thioalkalispira spp. and Thioalkalimicrobium spp. were unique to one environmental sample ( Vavourakis et al., 2019 ).
Four M&A literature samples were found to have elevated abundances (>10%) of non-c sox dominant SOB including three solid samples and one water sample ( Figure 4Bii ). Non-c sox dominant SOB abundances of the M&A literature samples were found to be highest in the only non-mining sample (13 SPM Swine Farm H 2 S Bioscrubber System; Figure 4Bii ). Three of the four M&A literature samples with elevated SOB abundances fell between pH ~6.5 and ~ 8, covering a similar range to the samples included in this study ( Figure 4Bi ). Among the seven M&A literature samples displaying elevated (>10%) c sox dominant SOB abundances, five were found to occur within a pH range of ~5 and ~ 7 ( Figure 4Bii ); a slightly higher upper range compared to this study’s TI results (< pH 6.5; Figure 4Aii ). The highest c sox dominant SOB abundances in M&A literature samples were identified in samples at pH ~5 and ~ 7 ( Figure 4Bii ) though no data between those two pH values are presently available in the literature. At pH >8, there were two M&A literature samples which both showed low abundances of both c sox dominant and non-c sox dominant SOB ( Figures 4Bi,ii ), aligned with the pattern of low SOB abundance at more alkaline pH observed in this study’s TI samples. Samples below pH 4 (closer to acid mine drainage conditions) from the M&A literature samples had very low SOB abundances ( Figures 4Bi,ii ) in both solid and water samples.
Differences observed between the M&A literature data and this study’s TI data may reflect differences in S substrate availability due to the types or locations of samples included. The M&A mining-related solid sample’s primary source of sulfur would be sulfide-containing ores whereas SOI, aqueous dissolved species, prevalent in the TI waters investigated here, would be limited in solid samples. Solid tailings and feed samples may similarly exhibit different patterns of SOB community structure and abundance and associated sulfur oxidation genes due to these differences in available sulfur species, as only one of the three main pathways highlighted here ( sox ) utilizes sulfide ( Klatt and Polerecky, 2015 ). This may account for the elevated c sox dominant SOB abundances observed in some of the circumneutral solid samples included in Figure 4Bii . Extrapolating the pattern identified in this study’s samples to other anthropogenically impacted environments would require more data (both genus level abundance and pH data) for TI water caps which are not currently available. However, these comparative results, across a range of systems where data are available, show some consistencies in patterns with those observed for base metal TI wastewaters, namely low SOB abundances at elevated pH values (pH >8) and similar pH ranges for the peak functional pathway abundances of both non-c sox dominant (pH ~6.5 to ~8.5) and c sox dominant (~4.5 to ~7) SOB ( Figures 4Bi,ii ).
The environmental literature samples that fell between pH ~6.5 and ~ 8.5 had elevated non-c sox dominant SOB abundances ( Figure 4Ci ) consistent with results for this study’s TI waters. No environmental literature samples were found between pH ~5 and ~ 6.5 precluding a direct comparison to the peak c sox dominant SOB abundances observed for TI here. Similar to the observed TI results here, however, three environmental literature samples (two samples <pH ~5 and one sample > pH ~8.5) had lower abundances of non-c sox dominant SOB than those within the more circum-neutral range (pH ~6.5 – ~8.5) ( Figure 4Ci ). The environmental literature group of samples was the only group where all samples had >1% non-c sox dominant SOB ( Figure 4Ci ). Unlike the previous two groups of samples, none of the environmental samples had high abundances of c sox dominant SOB (all <20% c sox dominant SOB abundance; Figure 4Cii ). This may reflect typically much lower environmental SOI concentrations ( O’Brien and Birkner, 1977 ) and therefore metabolisms that exhaust SOI by completely oxidizing them to SO 4 2- (i.e., c sox ) may not be favoured over those that disproportionate or recycle SOI such as the S 4 I or r dsr pathways (ie. non-c sox dominant SOB). The divergence of mining impacted environments (both in this study (oxic TI wastewaters) and literature data) from natural environments suggests a specialized mining specific microbiome with higher abundance(s) of c sox dominant SOB favoured by higher concentrations of S species in these contexts.
3.5 Factors influencing microbial community structure and function
The i sox pathway or the i sox + r dsr pathway generates more ATP and is more efficient due to energy conservation via S 0 production and storage ( Klatt and Polerecky, 2015 ). While energy efficiency is important, it is not the sole determinant of success in any given environment ( Klatt and Polerecky, 2015 ). This is exemplified across the diverse set of environments presented here, where both the i sox or i sox + rdsr pathway, as well as the c sox pathway appear at different times. Microbial success can be determined by growth rate, which considers both the substrate uptake rate and the growth yield ( Klatt and Polerecky, 2015 ). Both growth yield and efficiency are directly related, but lower efficiency (such as that associated with c sox ) can be made up for by speed, resulting in equal or greater success ( Sorokin and Kuenen, 2005 ; Klatt and Polerecky, 2015 ). Whaley-Martin et al. (2023) provided laboratory enrichment data from the same four mines included in this study to showcase that c sox SOB ( Halothiobacillus spp. dominant) enrichments exhibit a considerably faster S 2 O 3 2- oxidation rate compared to the enrichments with r dsr containing SOB. Growth strategies which rely on speed are only likely to dominate in environments with unlimited substrates ( Klatt and Polerecky, 2015 ). Though mining and other industrial environments technically do not have unlimited SOI, there are consistent replenishments of SOI through tailings additions or continuous wastewater treatment/flow. The observed proliferation of SOB with the c sox pathway in mining and industrial contexts ( Figures 4Aii,Bii ), aligns with their demonstrated capability for rapid S 2 O 3 2- oxidation under primarily oxic conditions. This highlights their competitive advantage in these substrate-rich environments where consistent SOI inputs help sustain their growth. The transition from non-c sox dominant SOB (i sox or i sox + r dsr ) to c sox dominant SOB occurs within the range of 0.1 to 0.3 mmol/L [S 2 O 3 2- ] ( Supplementary Figure S4A ), suggesting a potential SOI threshold for the initiation of this metabolic shift. The associated pH decrease with the increased presence of c sox dominant SOB may occur as a by-product of the activity of the c sox pathway which produces SO 4 2- and acidity and may slowly decrease pH. Further, under natural environmental conditions, where SOI are not as readily replenished, we do not see the proliferation of the c sox pathway ( Figure 4Cii ).
Across the four TIs investigated here, evidence of pH and [S 2 O 3 2- ] dependent occurrence of all four key metabolic pathways, c sox pathway, S 4 I pathway, i sox pathway, and r dsr pathway emerged ( Figure 5 ). pH partitioned three of these pathways: namely c sox pathway dominated at lower pH values (pH ~5 to ~6.5) while the i sox and r dsr pathways were more prevalent at circumneutral pH values (pH ~6.5 to ~8.5; Figure 5 ). The shift from circumneutral to more acidic pH may represent the onset of a positive feedback loop led by c sox dominant SOB such as Halothiobacillus spp. which has previously been implicated in catalyzing the shift to net acid generation in laboratory scale experiments ( Whaley-Martin et al., 2019 ). The c sox pathway, responsible for the direct oxidation of S 2 O 3 2- to SO 4 2- , which generates more acidity than the S 4 I, i sox , or r dsr pathway results in decreasing [S 2 O 3 2- ] and increasing [H + ]/[SO 4 2- ] values ( Figure 5 ). Uniquely, portions of the S 4 I pathway, namely the tsdA gene, were observed in SOB genera from across the entire pH range ( Figure 5 ).
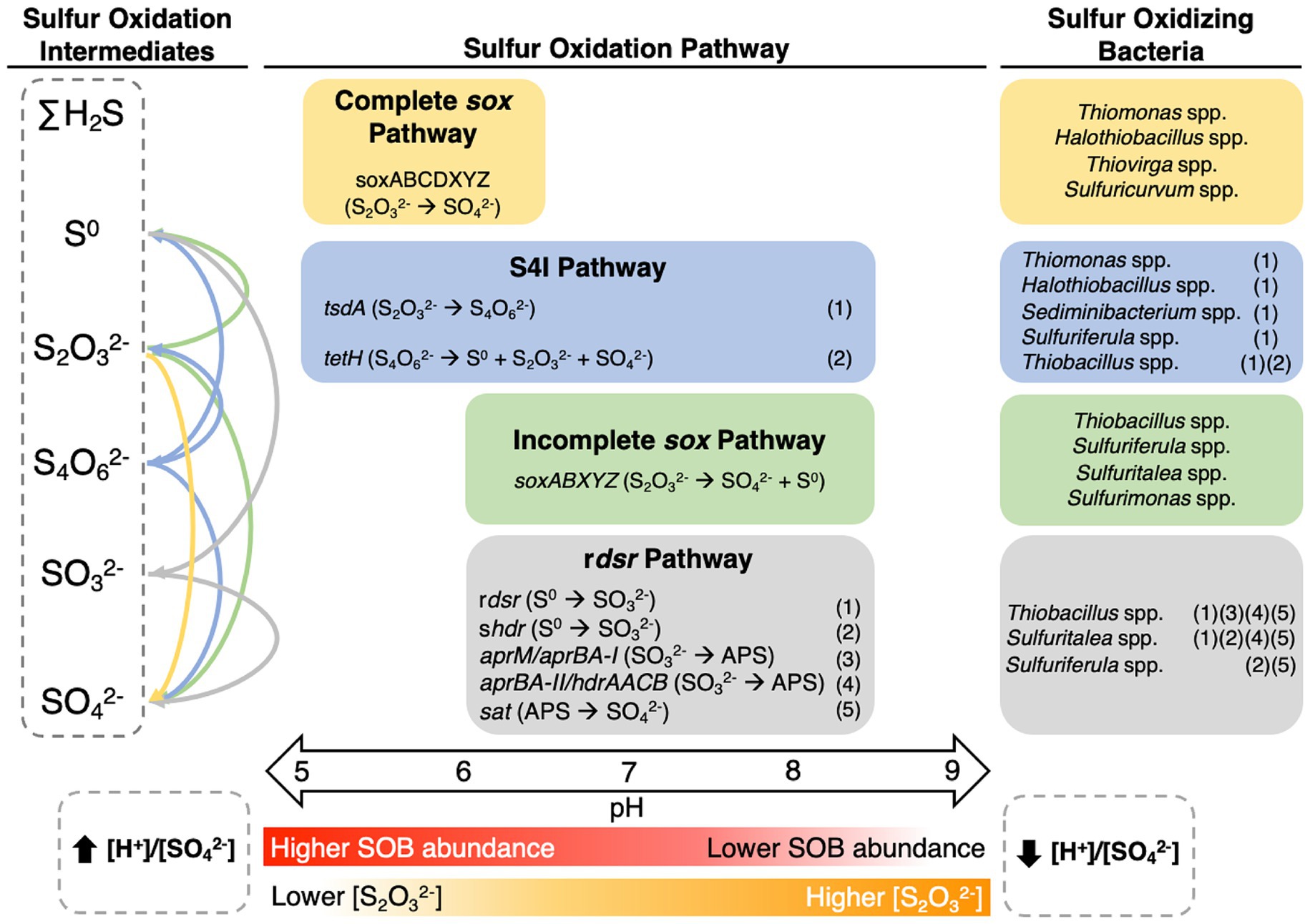
Figure 5 . Conceptual model of pH bound metabolic activity with the associated responsible genera and geochemical pathway and outcomes in metal mining TI waters.
4 Conclusion
Results here reveal new insights into the interplay between pH, SOB community composition and function (c sox dominant vs. non-c sox dominant), S cycling and acidity generation within primarily oxic mine TI waters over seasonal, annual, and mine operation scales. Our results highlight two key pH dependent niches characterized by the presence of either (i) c sox dominant SOB (e.g., Thiomonas spp., Halothiobacillus spp.) associated with lower pH values and lower [S 2 O 3 2- ] or (ii) non-c sox dominant SOB (i sox and/or r dsr pathways; e.g. Thiobacillus spp., Sulfuriferula spp.) associated with more circumneutral pH conditions and higher [S 2 O 3 2- ] ( Figure 5 ). The presence of the first part of the S 4 I pathway ( tsdA ; S 2 O 3 2- to S 4 O 6 2- ) was ubiquitous across pH and [S 2 O 3 2- ] niches; while possible subsequent processing of S 4 O 6 2- via tetH which can lead to S 0 , and S 2 O 3 2- regeneration, as well as SO 4 2- , was limited to Thiobacillus spp., observed more prevalently in circumneutral pH values ( Figure 5 ). Extrapolation of these results to broader environments via comparative analysis using M&A and environmental literature data revealed a specialized mining SOB microbiome characterized by elevated abundances of c sox dominant SOB. Elevated SOI concentrations, typical of mining environments, support the establishment and sustainment of c sox dominant communities unique to these environments. Our comprehensive study into SOB community dynamics, sulfur oxidation pathways, and the influence of geochemical and physicochemical factors in mining impacted waters, highlight the importance of thiosulfate availability, and pH constraints on sulfur oxidizing metabolism under oxic conditions, prevalent in TI contexts. This study highlights opportunities to manipulate TI SOB communities through pH adjustment and/or [S 2 O 3 2- ] management, offering potential avenues to reduce the risk of SOI discharge into receiving waters.
Data availability statement
The 16S rRNA sequencing data generated from this study have been deposited in the NCBI database under accession codes PRJNA510600, PRJNA882497, and PRJNA930015. The metagenome-assembled genomes generated in this study are available at ggKbase.berkeley.edu via https://ggkbase.berkeley.edu/SOB_across_mines/organisms .
Author contributions
LT: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, Resources, Software. KW-M: Investigation, Methodology, Project administration, Writing – review & editing. L-XC: Data curation, Formal analysis, Software, Writing – review & editing. TC: Investigation, Methodology, Project administration, Supervision, Writing – review & editing. JA: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. CJ: Methodology, Resources, Writing – review & editing. JK: Methodology, Resources, Writing – review & editing. LR: Methodology, Resources, Writing – review & editing. HS: Methodology, Resources, Writing – review & editing. JB: Conceptualization, Funding acquisition, Resources, Writing – review & editing. SA: Resources, Writing – review & editing, Methodology. LW: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Research activities and personnel were supported by Genome Canada Large Scale Applied Research Program 2015 (Grant Number OGI-124) and Ontario Research Fund–Research Excellence (Round 8).
Acknowledgments
The authors thank Laura Rossi for her laboratory assistance on this work. Additional thanks go out to on-site mine personnel who supported and aided in sample collection and processing including Stephanie Marshall, Sam McGarry, Nicole Gisby, Shirley Neault, Peter Mercer, Sean Breen, Landice Yestrau, Lana Leclerc, Ryan Trudeau, and Joel Nilsen. Research was made possible through the support of the Genome Canada Large Scale Applied Research Program and Ontario Research Fund – Research Excellence grants awarded to LW. The National Sciences and Engineering Research Council (NSERC) CREATE Mine of Knowledge Graduate Travel and Research Grants, Northern Scientific Training Program Travel Grant and QEII-GSST are also gratefully acknowledged.
Conflict of interest
LR and HS were employed by EcoReg Solutions.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1426584/full#supplementary-material
1. ^ ggkbase.berkeley.edu
Akcil, A., and Koldas, S. (2006). Acid mine drainage (AMD): causes, treatment and case studies. J. Clean. Prod. 14, 1139–1145. doi: 10.1016/J.JCLEPRO.2004.09.006
Crossref Full Text | Google Scholar
Anantharaman, K., Brown, C. T., Hug, L. A., Sharon, I., Castelle, C. J., Probst, A. J., et al. (2016). Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system. Nat. Commun. 7:13219. doi: 10.1038/ncomms13219
Apweiler, R., Bairoch, A., Wu, C. H., Barker, W. C., Boeckmann, B., Ferro, S., et al. (2004). UniProt: the universal protein knowledgebase. Nucleic Acids Res. 32, D115–D119. doi: 10.1093/NAR/GKH131
Arce-Rodríguez, A., Puente-Sánchez, F., Avendaño, R., Martínez-Cruz, M., de Moor, J. M., Pieper, D. H., et al. (2019). Thermoplasmatales and sulfur-oxidizing bacteria dominate the microbial community at the surface water of a CO 2-rich hydrothermal spring located in Tenorio volcano national park. Costa Rica. Extremophiles 23, 177–187. doi: 10.1007/s00792-018-01072-6
Auld, R. R., Mykytczuk, N. C. S., Leduc, L. G., and Merritt, T. J. S. (2017). Seasonal variation in an acid mine drainage microbial community. Can. J. Microbiol. 63, 137–152. doi: 10.1139/cjm-2016-0215
Bak, F., and Pfennig, N. (1987). Chemolithotrophic growth of Desulfovibrio sulfodismutans sp. nov. by disproportionation of inorganic sulfur compounds. Arch. Microbiol. 147, 184–189. doi: 10.1007/BF00415282
Baker, B. J., and Banfield, J. F. (2003). Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 44, 139–152. doi: 10.1016/S0168-6496(03)00028-X
Bamford, V. A., Bruno, S., Rasmussen, T., Appia-Ayme, C., Cheesman, M. R., Berks, B. C., et al. (2002). Structural basis for the oxidation of thiosulfate by a sulfur cycle enzyme. EMBO J. 21, 5599–5610. doi: 10.1093/EMBOJ/CDF566
Bartram, A. K., Lynch, M. D. J., Stearns, J. C., Moreno-Hagelsieb, G., and Neufeld, J. D. (2011). Generation of multimillion-sequence 16S rRNA gene libraries from complex microbial communities by assembling paired-end Illumina reads. Appl. Environ. Microbiol. 77, 3846–3852. doi: 10.1128/AEM.02772-10
Bates, S. T., Berg-Lyons, D., Caporaso, J. G., Walters, W. A., Knight, R., and Fierer, N. (2010). Examining the global distribution of dominant archaeal populations in soil. ISME J. 5, 908–917. doi: 10.1038/ismej.2010.171
Bernier, L., and Warren, L. A. (2007). Geochemical diversity in S processes mediated by culture-adapted and environmental-enrichments of Acidithiobacillus spp. Geochim. Cosmochim. Acta 71, 5684–5697. doi: 10.1016/J.GCA.2007.08.010
Bond, P. L., Druschel, G. K., and Banfield, J. F. (2000). Comparison of acid mine drainage microbial communities in physically and geochemically distinct ecosystems. Appl. Environ. Microbiol. 66, 4962–4971. doi: 10.1128/AEM.66.11.4962-4971.2000
Brito, J. A., Denkmann, K., Pereira, I. A. C., Archer, M., and Dahl, C. (2015). Thiosulfate dehydrogenase (TsdA) from Allochromatium vinosum . J. Biol. Chem. 290, 9222–9238. doi: 10.1074/jbc.m114.623397
Brown, C. T., Hug, L. A., Thomas, B. C., Sharon, I., Castelle, C. J., Singh, A., et al. (2015). Unusual biology across a group comprising more than 15% of domain Bacteria. Nature 523, 208–211. doi: 10.1038/nature14486
Bushnell, B., Rood, J., and Singer, E. (2017). BBMerge–accurate paired shotgun read merging via overlap. PLoS One 12:e0185056. doi: 10.1371/JOURNAL.PONE.0185056
Camacho, D., Frazao, R., Fouillen, A., Nanci, A., Lang, B. F., Apte, S. C., et al. (2020a). New insights into Acidithiobacillus thiooxidans sulfur metabolism through coupled gene expression, solution chemistry, microscopy, and spectroscopy analyses. Front. Microbiol. 11:411. doi: 10.3389/fmicb.2020.00411
Camacho, D., Jessen, G. L., Mori, J. F., Apte, S. C., Jarolimek, C. V., and Warren, L. A. (2020b). Microbial succession signals the initiation of acidification in mining wastewaters. Mine Water Environ. 39, 669–683. doi: 10.1007/S10230-020-00711-9/FIGURES/5
Campa, M. F., Chen See, J. R., Unverdorben, L. V., Wright, O. G., Roth, K. A., Niles, J. M., et al. (2022). Geochemistry and multiomics data differentiate streams in Pennsylvania based on unconventional oil and gas activity. Microbiol. Spect. 10:22. doi: 10.1128/spectrum.00770-22
Canfield, D. E., and Farquhar, J. (2009). Animal evolution, bioturbation, and the sulfate concentration of the oceans. Proc. Natl. Acad. Sci. 106, 8123–8127. doi: 10.1073/PNAS.0902037106
Cao, X., Koch, T., Steffens, L., Finkensieper, J., Zigann, R., Cronan, J. E., et al. (2018). Lipoate-binding proteins and specific lipoate-protein ligases in microbial sulfur oxidation reveal an atpyical role for an old cofactor. eLife 7:e37439. doi: 10.7554/ELIFE.37439
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Huntley, J., Fierer, N., et al. (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. Int. Soc. Microb. Ecol. J. 6, 1621–1624. doi: 10.1038/ismej.2012.8
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Lozupone, C. A., Turnbaugh, P. J., et al. (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 108, 4516–4522. doi: 10.1073/pnas.1000080107
Chan, P. P., and Lowe, T. M. (2019). tRNAscan-SE: searching for tRNA genes in genomic sequences. Methods Mol. Biol. 1962, 1–14. doi: 10.1007/978-1-4939-9173-0_1
Chen, L. X., Anantharaman, K., Shaiber, A., Murat Eren, A., and Banfield, J. F. (2020). Accurate and complete genomes from metagenomes. Genome Res. 30, 315–333. doi: 10.1101/GR.258640.119
Chen, L., Li, J., Chen, Y., Huang, L., Hua, Z., Hu, M., et al. (2013). Shifts in microbial community composition and function in the acidification of a lead/zinc mine tailings. Environ. Microbiol. 15, 2431–2444. doi: 10.1111/1462-2920.12114
Dahl, C., Franz, B., Hensen, D., Kesselheim, A., and Zigann, R. (2013). Sulfite oxidation in the purple sulfur bacterium Allochromatium vinosum : identification of SoeABC as a major player and relevance of SoxYZ in the process. Microbiology 159, 2626–2638. doi: 10.1099/mic.0.071019-0
Dam, B., Mandal, S., Ghosh, W., Das Gupta, S. K., and Roy, P. (2007). The S4-intermediate pathway for the oxidation of thiosulfate by the chemolithoautotroph Tetrathiobacter kashmirensis and inhibition of tetrathionate oxidation by sulfite. Res. Microbiol. 158, 330–338. doi: 10.1016/J.RESMIC.2006.12.013
Dold, B. (2014). Evolution of acid mine drainage formation in Sulphidic mine tailings. Fortschr. Mineral. 4, 621–641. doi: 10.3390/MIN4030621
Druschel, G. K., Hamers, R. J., and Banfield, J. F. (2003). Kinetics and mechanism of polythionate oxidation to sulfate at low pH by O2 and Fe3+. Geochim. Cosmochim. Acta 67, 4457–4469. doi: 10.1016/S0016-7037(03)00388-0
Durazzi, F., Sala, C., Castellani, G., Manfreda, G., Remondini, D., and De Cesare, A. (2021). Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci. Rep. 11, 1–10. doi: 10.1038/s41598-021-82726-y
Edgar, R. C., and Bateman, A. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. doi: 10.1093/BIOINFORMATICS/BTQ461
Edwards, K. J., Gihring, T. M., and Banfield, J. F. (1999). Seasonal variations in microbial populations and environmental conditions in an extreme acid mine drainage environment. Appl. Environ. Microbiol. 65, 3627–3632. doi: 10.1128/AEM.65.8.3627-3632.1999
Elberling, B., and Damgaard, L. R. (2001). Microscale measurements of oxygen diffusion and consumption in subaqueous sulfide tailings. Geochim. Cosmochim. Acta 65, 1897–1905. doi: 10.1016/S0016-7037(01)00574-9
Foucher, S., Battaglia-Brunet, F., Ignatiadis, I., and Morin, D. (2001). Treatment by sulfate-reducing bacteria of Chessy acid-mine drainage and metals recovery. Chem. Eng. Sci. 56, 1639–1645. doi: 10.1016/S0009-2509(00)00392-4
Friedrich, C. G., Bardischewsky, F., Rother, D., Quentmeier, A., and Fischer, J. (2005). Prokaryotic sulfur oxidation. Curr. Opin. Microbiol. 8, 253–259. doi: 10.1016/J.MIB.2005.04.005
Friedrich, C. G., Quentmeier, A., Bardischewsky, F., Rother, D., Kraft, R., Kostka, S., et al. (2000). Novel genes coding for lithotrophic sulfur oxidation of Paracoccus pantotrophus GB17. J. Bacteriol. 182, 4677–4687. doi: 10.1128/jb.182.17.4677-4687.2000
Friedrich, C. G., Rother, D., Bardischewsky, F., Ouentmeier, A., and Fischer, J. (2001). Oxidation of reduced inorganic sulfur compounds by Bacteria: emergence of a common mechanism? Appl. Environ. Microbiol. 67, 2873–2882. doi: 10.1128/AEM.67.7.2873-2882.2001
Frigaard, N. U., and Dahl, C. (2008). Sulfur metabolism in phototrophic sulfur Bacteria. Adv. Microb. Physiol. 54, 103–200. doi: 10.1016/S0065-2911(08)00002-7
Ghosh, W., and Dam, B. (2009). Biochemistry and molecular biology of lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea. FEMS Microbiol. Rev. 33, 999–1043. doi: 10.1111/J.1574-6976.2009.00187.X
Grabarczyk, D. B., and Berks, B. C. (2017). Intermediates in the sox sulfur oxidation pathway are bound to a sulfane conjugate of the carrier protein SoxYZ. PLoS One 12:173395. doi: 10.1371/JOURNAL.PONE.0173395
Han, Y., and Perner, M. (2015). The globally widespread genus Sulfurimonas: versatile energy metabolisms and adaptations to redox clines. Front. Microbiol. 6:989. doi: 10.3389/fmicb.2015.00989
Handley, K. M., Bartels, D., O’Loughlin, E. J., Williams, K. H., Trimble, W. L., Skinner, K., et al. (2014). The complete genome sequence for putative H2- and S-oxidizer Candidatus Sulfuricurvum sp., assembled de novo from an aquifer-derived metagenome. Environ. Microbiol. 16, 3443–3462. doi: 10.1111/1462-2920.12453
Haosagul, S., Prommeenate, P., Hobbs, G., and Pisutpaisal, N. (2020). Sulfide-oxidizing bacteria community in full-scale bioscrubber treating H2S in biogas from swine anaerobic digester. Renew. Energy 150, 973–980. doi: 10.1016/J.RENENE.2019.11.139
Hong, S., Bunge, J., Leslin, C., Jeon, S., and Epstein, S. S. (2009). Polymerase chain reaction primers miss half of rRNA microbial diversity. ISME J. 3, 1365–1373. doi: 10.1038/ISMEJ.2009.89
Houghton, J. L., Foustoukos, D. I., Flynn, T. M., Vetriani, C., Bradley, A. S., and Fike, D. A. (2016). Thiosulfate oxidation by Thiomicrospira thermophila : metabolic flexibility in response to ambient geochemistry. Environ. Microbiol. 18, 3057–3072. doi: 10.1111/1462-2920.13232
Hutt, L. P., Huntemann, M., Clum, A., Pillay, M., Palaniappan, K., Varghese, N., et al. (2017). Permanent draft genome of Thiobacillus thioparus DSM 505T, an obligately chemolithoautotrophic member of the Betaproteobacteria. Stand. Genomic Sci. 12, 1–8. doi: 10.1186/s40793-017-0229-3
Hyatt, D., Chen, G. L., LoCascio, P. F., Land, M. L., Larimer, F. W., and Hauser, L. J. (2010). Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformat. 11:119. doi: 10.1186/1471-2105-11-119
Johnson, D. B., and Hallberg, K. B. (2003). The microbiology of acidic mine waters. Res. Microbiol. 154, 466–473. doi: 10.1016/S0923-2508(03)00114-1
Kadnikov, V. V., Mardanov, A. V., Beletsky, A. V., Antsiferov, D. V., Kovalyova, A. A., Karnachuk, O. V., et al. (2019). Sulfur-oxidizing Bacteria dominate in the water from a flooded coal mine shaft in Kuzbass. Microbiology 88, 120–123. doi: 10.1134/S0026261719010107
Kanehisa, M., Furumichi, M., Tanabe, M., Sato, Y., and Morishima, K. (2017). KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 45, D353–D361. doi: 10.1093/NAR/GKW1092
Kanehisa, M., and Sato, Y. (2020). KEGG mapper for inferring cellular functions from protein sequences. Prot. Sci. 29, 28–35. doi: 10.1002/PRO.3711
Kang, D. D., Froula, J., Egan, R., and Wang, Z. (2015). MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ 2015:e1165. doi: 10.7717/peerj.1165
Kelly, D. P., Shergill, J. K., Lu, W. P., and Wood, A. P. (1997). Oxidative metabolism of inorganic sulfur compounds by bacteria. Antonie Van Leeuwenhoek 71, 95–107. doi: 10.1023/A:1000135707181
Kembel, S. W., Wu, M., Eisen, J. A., and Green, J. L. (2012). Incorporating 16S gene copy number information improves estimates of microbial diversity and abundance. PLoS Comput. Biol. 8:e1002743. doi: 10.1371/JOURNAL.PCBI.1002743
Kinnunen, P., Kyllönen, H., Kaartinen, T., Mäkinen, J., Heikkinen, J., and Miettinen, V. (2018). Sulphate removal from mine water with chemical, biological and membrane technologies. Water Sci. Technol. 2017, 194–205. doi: 10.2166/WST.2018.102
Klatt, J. M., and Polerecky, L. (2015). Assessment of the stoichiometry and efficiency of CO2 fixation coupled to reduced sulfur oxidation. Front. Microbiol. 6:484. doi: 10.3389/fmicb.2015.00484
Koch, T., and Dahl, C. (2018). A novel bacterial sulfur oxidation pathway provides a new link between the cycles of organic and inorganic sulfur compounds. ISME J. 12, 2479–2491. doi: 10.1038/s41396-018-0209-7
Korehi, H., Blöthe, M., and Schippers, A. (2014). Microbial diversity at the moderate acidic stage in three different sulfidic mine tailings dumps generating acid mine drainage. Res. Microbiol. 165, 713–718. doi: 10.1016/J.RESMIC.2014.08.007
Kuang, J., Huang, L., He, Z., Chen, L., Hua, Z., Jia, P., et al. (2016). Predicting taxonomic and functional structure of microbial communities in acid mine drainage. Int. Soc. Microb. Ecol. J. 10, 1527–1539. doi: 10.1038/ismej.2015.201
Lacelle, D., Doucet, A., Clark, I. D., and Lauriol, B. (2007). Acid drainage generation and seasonal recycling in disturbed permafrost near Eagle Plains, northern Yukon territory Canada. Chem. Geol. 243, 157–177. doi: 10.1016/J.CHEMGEO.2007.05.021
Lahme, S., Callbeck, C. M., Eland, L. E., Wipat, A., Enning, D., Head, I. M., et al. (2020). Comparison of sulfide-oxidizing Sulfurimonas strains reveals a new mode of thiosulfate formation in subsurface environments. Environ. Microbiol. 22, 1784–1800. doi: 10.1111/1462-2920.14894
Langmead, B., and Salzberg, S. L. (2012). Fast gapped-read alignment with bowtie 2. Nat. Methods 9, 357–359. doi: 10.1038/nmeth.1923
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/BIOINFORMATICS/BTP352
Liljeqvist, M., Sundkvist, J. E., Saleh, A., and Dopson, M. (2011). Low temperature removal of inorganic sulfur compounds from mining process waters. Biotechnol. Bioeng. 108, 1251–1259. doi: 10.1002/BIT.23057
Lin, K. H., Liao, B. Y., Chang, H. W., Huang, S. W., Chang, T. Y., Yang, C. Y., et al. (2015). Metabolic characteristics of dominant microbes and key rare species from an acidic hot spring in Taiwan revealed by metagenomics. BMC Genom. 16, 1–16. doi: 10.1186/s12864-015-2230-9
Lopes, A. R., Madureira, D., Diaz, A., Santos, S., Vila, M. C., and Nunes, O. C. (2020). Characterisation of bacterial communities from an active mining site and assessment of its potential metal solubilising activity. J. Environ. Chem. Eng. 8:104495. doi: 10.1016/J.JECE.2020.104495
Louca, S., Polz, M. F., Mazel, F., Albright, M. B. N., Huber, J. A., O’Connor, M. I., et al. (2018). Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2, 936–943. doi: 10.1038/s41559-018-0519-1
Loy, A., Duller, S., Baranyi, C., Mußmann, M., Ott, J., Sharon, I., et al. (2009). Reverse dissimilatory sulfite reductase as phylogenetic marker for a subgroup of sulfur-oxidizing prokaryotes. Environ. Microbiol. 11, 289–299. doi: 10.1111/J.1462-2920.2008.01760.X
Makhija, R., and Hitchen, A. (1979). The titrimetric determination of sulphate, thiosulphate and polythionates in mining effluents. Anal. Chim. Acta 105, 375–382. doi: 10.1016/S0003-2670(01)83769-7
Martin, M. (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12. doi: 10.14806/EJ.17.1.200
Meier, D. V., Pjevac, P., Bach, W., Hourdez, S., Girguis, P. R., Vidoudez, C., et al. (2017). Niche partitioning of diverse sulfur-oxidizing bacteria at hydrothermal vents. ISME J. 11, 1545–1558. doi: 10.1038/ismej.2017.37
Meyer, B., and Kuever, J. (2007). Molecular analysis of the distribution and phylogeny of dissimilatory adenosine-5′-phosphosulfate reductase-encoding genes (aprBA) among sulfur-oxidizing prokaryotes. Microbiology 153, 3478–3498. doi: 10.1099/MIC.0.2007/008250-0
Meziti, A., Nikouli, E., Hatt, J. K., Konstantinidis, K. T., and Kormas, K. A. (2021). Time series metagenomic sampling of the Thermopyles, Greece, geothermal springs reveals stable microbial communities dominated by novel sulfur-oxidizing chemoautotrophs. Environ. Microbiol. 23, 3710–3726. doi: 10.1111/1462-2920.15373
Miettinen, H., Bomberg, M., Le, T. M. K., and Kinnunen, P. (2021). Identification and metabolism of naturally prevailing microorganisms in zinc and copper mineral processing. Fortschr. Mineral. 11:30. doi: 10.3390/MIN11020156
Miranda-Trevino, J. C., Pappoe, M., Hawboldt, K., and Bottaro, C. (2013). The importance of Thiosalts speciation: review of analytical methods, kinetics, and treatment. Crit. Rev. Environ. Sci. Technol. 43, 2013–2070. doi: 10.1080/10643389.2012.672047
Neukirchen, S., and Sousa, F. L. (2021). DiSCo: a sequence-based type-specific predictor of Dsr-dependent dissimilatory Sulphur metabolism in microbial data. Microbial Genom. 7:603. doi: 10.1099/MGEN.0.000603
Nguyen, P. M., Do, P. T., Pham, Y. B., Doan, T. O., Nguyen, X. C., Lee, W. K., et al. (2022). Roles, mechanism of action, and potential applications of sulfur-oxidizing bacteria for environmental bioremediation. Sci. Total Environ. 852:158203. doi: 10.1016/J.SCITOTENV.2022.158203
Nosalova, L., Piknova, M., Kolesarova, M., and Pristas, P. (2023). Cold Sulfur Springs—neglected niche for autotrophic sulfur-oxidizing Bacteria. Microorganisms 11:1436. doi: 10.3390/MICROORGANISMS11061436
Nurk, S., Meleshko, D., Korobeynikov, A., and Pevzner, P. A. (2017). MetaSPAdes: a new versatile metagenomic assembler. Genome Res. 27, 824–834. doi: 10.1101/gr.213959.116
O’Brien, D. J., and Birkner, F. B. (1977). Kinetics of oxygenation of reduced sulfur species in aqueous solution. Environ. Sci. Technol. 11, 1114–1120. doi: 10.1021/es60135a009
Patwardhan, S., Foustoukos, D. I., Giovannelli, D., Yücel, M., and Vetriani, C. (2018). Ecological succession of sulfur-oxidizing epsilon- and gammaproteobacteria during colonization of a shallow-water gas vent. Front. Microbiol. 9:2970. doi: 10.3389/fmicb.2018.02970
Peng, Y., Leung, H. C. M., Yiu, S. M., and Chin, F. Y. L. (2012). IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 28, 1420–1428. doi: 10.1093/BIOINFORMATICS/BTS174
Philippot, P., Van Zuilen, M., Lepot, K., Thomazo, C., Farquhar, J., and Van Kranendonk, M. J. (2007). Early archaean microorganisms preferred elemental sulfur, not sulfate. Science 317, 1534–1537. doi: 10.1126/science.1145861
Pott, A. S., and Dahl, C. (1998). Sirohaem sulfite reductase and other proteins encoded by genes at the dsr locus of Chromatium vinosum are involved in the oxidation of intracellular sulfur. Microbiology 144, 1881–1894. doi: 10.1099/00221287-144-7-1881
Purcell, A. M., Mikucki, J. A., Achberger, A. M., Alekhina, I. A., Barbante, C., Christner, B. C., et al. (2014). Microbial sulfur transformations in sediments from subglacial Lake Whillans. Front. Microbiol. 5:594. doi: 10.3389/FMICB.2014.00594
Quentmeier, A., Kraft, R., Kostka, S., Klockenkämper, R., and Friedrich, C. G. (2000). Characterization of a new type of sulfite dehydrogenase from Paracoccus pantotrophus GB17. Arch. Microbiol. 173, 117–125. doi: 10.1007/s002039900118
Reigstad, L. J., Jorgensen, S. L., Lauritzen, S. E., Schleper, C., and Urich, T. (2011). Sulfur-oxidizing chemolithotrophic proteobacteria dominate the microbiota in high arctic thermal springs on Svalbard. Astrobiology 11, 665–678. doi: 10.1089/AST.2010.0551
Rethmeier, J., Rabenstein, A., Langer, M., and Fischer, U. (1997). Detection of traces of oxidized and reduced sulfur compounds in small samples by combination of different high-performance liquid chromatography methods. J. Chromatogr. A 760, 295–302. doi: 10.1016/S0021-9673(96)00809-6
Sato, I., Shimatani, K., Fujita, K., Abe, T., Shimizu, M., Fujii, T., et al. (2011). Glutathione reductase/glutathione is responsible for cytotoxic elemental sulfur tolerance via polysulfide shuttle in fungi. J. Biol. Chem. 286, 20283–20291. doi: 10.1074/jbc.M111.225979
Seth, N., Vats, S., Lakhanpaul, S., Arafat, Y., Mazumdar-Leighton, S., Bansal, M., et al. (2024). Microbial community diversity of an integrated constructed wetland used for treatment of sewage. Front. Microbiol. 15:1355718. doi: 10.3389/FMICB.2024.1355718
Silva, A. M., Lima, R. M. F., and Leão, V. A. (2012). Mine water treatment with limestone for sulfate removal. J. Hazard. Mater. 221–222, 45–55. doi: 10.1016/J.JHAZMAT.2012.03.066
Sorokin, D. Y., and Kuenen, J. G. (2005). Haloalkaliphilic sulfur-oxidizing bacteria in soda lakes. FEMS Microbiol. Rev. 29, 685–702. doi: 10.1016/J.FEMSRE.2004.10.005
Sorokin, D. Y., Lysenko, A. M., Mityushina, L. L., Tourova, T. P., Jones, B. E., Rainey, F. A., et al. (2001). Thioalkalimicrobium aerophilum gen. Nov., sp. nov. and Thioalkalimicrobium sibericum sp. nov., and Thioalkalivibrio versutus gen. Nov., sp. nov., Thioalkalivibrio nitratis sp. nov. and Thioalkalivibrio denitrificans sp. nov., novel obligately alkaliphilic and obligately chemolithoautotrophic sulfur-oxidizing bacteria from soda lakes. Int. J. Syst. Evol. Microbiol. 51, 565–580. doi: 10.1099/00207713-51-2-565
Suzek, B. E., Huang, H., McGarvey, P., Mazumder, R., and Wu, C. H. (2007). UniRef: comprehensive and non-redundant UniProt reference clusters. Bioinformatics 23, 1282–1288. doi: 10.1093/BIOINFORMATICS/BTM098
Suzuki, M. T., and Giovannoni, S. J. (1996). Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 62, 625–630. doi: 10.1128/AEM.62.2.625-630.1996
Thamdrup, B., Fossing, H., and Jørgensen, B. B. (1994). Manganese, iron and sulfur cycling in a coastal marine sediment, Aarhus bay Denmark. Geochim. Cosmochim. Acta 58, 5115–5129. doi: 10.1016/0016-7037(94)90298-4
van Vliet, D. M., von Meijenfeldt, F. A. B., Dutilh, B. E., Villanueva, L., Sinninghe Damsté, J. S., Stams, A. J. M., et al. (2021). The bacterial sulfur cycle in expanding dysoxic and euxinic marine waters. Environ. Microbiol. 23, 2834–2857. doi: 10.1111/1462-2920.15265
Vavourakis, C. D., Mehrshad, M., Balkema, C., Van Hall, R., Andrei, A. Ş., Ghai, R., et al. (2019). Metagenomes and metatranscriptomes shed new light on the microbial-mediated sulfur cycle in a Siberian soda lake. BMC Biol. 17, 1–20. doi: 10.1186/s12915-019-0688-7
Veith, A., Botelho, H. M., Kindinger, F., Gomes, C. M., and Kletzin, A. (2012). The sulfur oxygenase reductase from the mesophilic bacterium Halothiobacillus neapolitanus is a highly active Thermozyme. J. Bacteriol. 194:677. doi: 10.1128/JB.06531-11
Vincent, S. G. T., Jennerjahn, T., and Ramasamy, K. (2021). “Biogeocycling of nutrients (C, N, P, S, and Fe) and implications on greenhouse gas emissions” in Microbial communities in coastal sediments . eds. S. G. T. Vincent, T. C. Jennerjahn, and K. Ramasamy (Amsterdam: Elsevier), 119–145.
Google Scholar
Wang, S., Jiang, L., Hu, Q., Cui, L., Zhu, B., Fu, X., et al. (2021). Characterization of Sulfurimonas hydrogeniphila sp. nov., a novel bacterium predominant in Deep-Sea hydrothermal vents and comparative genomic analyses of the genus Sulfurimonas. Front. Microbiol. 12:626705. doi: 10.3389/fmicb.2021.626705
Wang, S., Jiang, L., Xie, S., Alain, K., Wang, Z., Wang, J., et al. (2023). Disproportionation of inorganic sulfur compounds by mesophilic Chemolithoautotrophic Campylobacterota. MSystems 8:22. doi: 10.1128/MSYSTEMS.00954-22
Wang, Z. B., Li, Y. Q., Lin, J. Q., Pang, X., Liu, X. M., Liu, B. Q., et al. (2016). The two-component system RsrS-RsrR regulates the tetrathionate intermediate pathway for thiosulfate oxidation in Acidithiobacillus caldus . Front. Microbiol. 7:1755. doi: 10.3389/fmicb.2016.01755
Wang, R., Lin, J. Q., Liu, X. M., Pang, X., Zhang, C. J., Yang, C. L., et al. (2019). Sulfur oxidation in the acidophilic autotrophic Acidithiobacillus spp. Front. Microbiol. 9:3290. doi: 10.3389/fmicb.2018.03290
Watanabe, T., Kojima, H., and Fukui, M. (2014). Complete genomes of freshwater sulfur oxidizers Sulfuricella denitrificans skB26 and Sulfuritalea hydrogenivorans sk43H: genetic insights into the sulfur oxidation pathway of betaproteobacteria. Syst. Appl. Microbiol. 37, 387–395. doi: 10.1016/J.SYAPM.2014.05.010
Watanabe, T., Kojima, H., Umezawa, K., Hori, C., Takasuka, T. E., Kato, Y., et al. (2019). Genomes of neutrophilic sulfur-oxidizing chemolithoautotrophs representing 9 proteobacterial species from 8 genera. Front. Microbiol. 10:316. doi: 10.3389/fmicb.2019.00316
Whaley-Martin, K. J., Chen, L.-X., Nelson, T. C., Gordon, J., Kantor, R., Twible, L. E., et al. (2023). O2 partitioning of sulfur oxidizing bacteria drives acidity and thiosulfate distributions in mining waters. Nat. Commun. 14:2006. doi: 10.1038/s41467-023-37426-8
Whaley-Martin, K., Jessen, G. L., Nelson, T. C., Mori, J. F., Apte, S., Jarolimek, C., et al. (2019). The potential role of Halothiobacillus spp. in sulfur oxidation and acid generation in circum-neutral mine tailings reservoirs. Front. Microbiol. 10:297. doi: 10.3389/FMICB.2019.00297/BIBTEX
Whaley-Martin, K., Marshall, S., Nelson, T. E. C., Twible, L., Jarolimek, C. V., King, J. J., et al. (2020). A mass-balance tool for monitoring potential dissolved sulfur oxidation risks in mining impacted waters. Mine Water Environ. 39, 291–307. doi: 10.1007/s10230-020-00671-0
Xin, Y., Wang, Y., Zhang, H., Wu, Y., Xia, Y., Li, H., et al. (2023). Cupriavidus pinatubonensis JMP134 alleviates Sulfane sulfur toxicity after the loss of Sulfane dehydrogenase through oxidation by Persulfide dioxygenase and hydrogen sulfide release. Meta 13:218. doi: 10.3390/METABO13020218/S1
Yan, Y., Colenbrander Nelson, T. E., Twible, L., Whaley-Martin, K., Jarolimek, C. V., King, J. J., et al. (2022). Sulfur mass balance and speciation in the water cap during early-stage development in the first pilot pit lake in the Alberta Oil Sands. Environ. Chem. 19, 236–253. doi: 10.1071/EN22057
Yu, X., Li, Y., Wu, Y., Gao, H., Liu, W., Liu, H., et al. (2024). Seasonal changes of prokaryotic microbial community structure in Zhangjiayan reservoir and its response to environmental factors. Sci. Rep. 14:5513. doi: 10.1038/s41598-024-55702-5
Zander, U., Faust, A., Klink, B. U., De Sanctis, D., Panjikar, S., Quentmeier, A., et al. (2011). Structural basis for the oxidation of protein-bound sulfur by the sulfur cycle molybdohemo-enzyme sulfane dehydrogenase SoxCD. J. Biol. Chem. 286, 8349–8360. doi: 10.1074/jbc.M110.193631
Zarroca, M., Roqué, C., Linares, R., Salminci, J. G., and Gutiérrez, F. (2021). Natural acid rock drainage in alpine catchments: a side effect of climate warming. Sci. Total Environ. 778:146070. doi: 10.1016/J.SCITOTENV.2021.146070
Keywords: sulfur oxidizing bacteria (SOB), pH, tailings impoundments, sox genes, thiosulfate
Citation: Twible LE, Whaley-Martin K, Chen L-X, Colenbrander Nelson T, Arrey JLS, Jarolimek CV, King JJ, Ramilo L, Sonnenberg H, Banfield JF, Apte SC and Warren LA (2024) pH and thiosulfate dependent microbial sulfur oxidation strategies across diverse environments. Front. Microbiol . 15:1426584. doi: 10.3389/fmicb.2024.1426584
Received: 01 May 2024; Accepted: 18 June 2024; Published: 19 July 2024.
Reviewed by:
Copyright © 2024 Twible, Whaley-Martin, Chen, Colenbrander Nelson, Arrey, Jarolimek, King, Ramilo, Sonnenberg, Banfield, Apte and Warren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lesley A. Warren, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Visit our Popular Forums
- Monohull Sailboats
- Multihull Sailboats
- Powered Boats
- General Sailing
- Antares Yachts
- Fountaine Pajot
- Lagoon Catamarans
Cruising Business
- Boat Classifieds
- General Classifieds
- Crew Positions
- Commercial Posts
- Vendor Spotlight
Life Aboard a Boat
- Provisioning: Food & Drink
- Families, Kids, & Pets Afloat
- Recreation, Entertainment, & Fun
- Boat Ownership & Making a Living
- Liveaboard's Forum
Seamanship, Navigation & Boat Handling
- Seamanship & Boat Handling
- Training, Licensing, & Certification
- Health, Safety, & Related Gear
- Rules of the Road, Regulations, & Red Tape
Engineering & Systems
- Const. / Maint. / Refit
- Product / Service Reviews
- Electronics: Comms / AV
- Electrical: Batts / Gen / Solar
- Lithium Power Systems
- Engines & Propulsion
- Propellers & Drive Systems
- Plumbing / Fixtures
- Deck Hdw: Rigging / Sails
- Aux. Equipment & Dinghy
- Anchoring & Mooring
Photo Categories
- Member Galleries
- Life Onboard
- Sailing in the Wind
- Power Boats
- Cruising Destinations
- Maint. & Boat Building
- Marine Life
- Scuba Diving & Divers
- General Photos
Recent Photos

Listing Categories
- African Cats
- view more »
- Crew Wanted
- Crew Available
- Enhance Your Account
- Meet the Mods
- Meet the Advisors
- Signup for The Daily Cruiser Email




IMAGES
VIDEO
COMMENTS
Experience with a Pearson 10M. I'm the third owner of a 10M that I purchased in 1991, and used - until recently - to cruise the Maine coast. It's very stable and offers performance similar to boats 3-7 feet longer. The original construction was probably overdesigned, with very thick hull sections and a heavy rig.
Pearson 10M is a very nice boat that should serve you well. You should get very familiar with your boat before you venture out and get to know each and every system on it. This just can't be overemphasized, especially with regards to critical systems, such as water ingress points, pumps, electrical system, engine, steering, rigging, and so on.
967 posts · Joined 2007. #8 · Mar 30, 2010. Pearson 10M is an outstanding boat imho. My routinely beats much larger boats easily and is a dream to sail. Looking at it you'de think it was slow, 33 ft long 11 foot beam, heavy (13,000 lbs), but it has a PHRF rating of around 140. Not speedy, but a good handicap racer.
Join Date: Aug 2006. Location: Skagit City, WA. Posts: 25,644. Re: Pearson 10 Meter. Long waterline, plenty of beam, skeg rudder. That stuff sounds good. PEARSON 10M sailboat specifications and details on sailboatdata.com. You can probably tell if its a cored hull by looking inside cabinets etc.
Pearson 26 OD Mobile Yacht Club. Mar 31, 2016. #2. There is a 10 Meter in an adjacent marina that i have been eye balling for about a year. I have spoken to multiple people who have sailed on her, they all say that the boat performs incredibly well in winds over 14 knots but not so good in light air. There are a few things you need to look for ...
I am wondering if taking a 1975 Pearson 10 meter is seaworthy enough to sail off to the Caribbean.I had a Pearson 424 Cutter / Ketch and made a few trips from Miami to Newport R I. on the outside and she handled beautiful. But that is a different animal. An approx. 11 day continuous trip. I do take the 10 meter out on 20 kt.- 23 kt. winds and she seems stable.
The 10M came with several different engine installations. Most were powered by the Atomic Four gas engine. Diesels were optional with a Faryman 25hp in 75, a Westerbeake 20hp diesel in 76. the Faryman again in 77, and a Volvo 23hp (MD11) from 78-80. Available with a taller rig: I(IG): 46.00′ / 14.02m J: 14.20′ […]
That means a sun-shade for protection, updated stereo sound system, pedestal mounted GPS and cup holders, all within reach of your comfy wheel-helm. This is your time to make the most of the sailing season. So don't miss your chance to buy this magnificent yacht, the Pearson 10M. Contact Fred for arrangements and info: 3479273350.
My plan was to wait for another year or so before buying a sailboat, and was thinking something between 25 and 30 feet in the $15K-$30K range would suit me best, but the most important criteria for me is finding a boat as close to pristine as possible. Hence, my interest in this Pearson 10 meter.
The Pearson 10M is equipped with a fin keel. The fin keel is the most common keel and provides splendid manoeuvrability. The downside is that it has less directional stability than a long keel. The boat can only enter major marinas as the draft is about 1.80 - 1.90 meter (5.91 - 6.21 ft) dependent on the load.
Pearson 10 Meter Thread starter thomm124; Start date Sep 20, 2017; This site may earn a commission from merchant affiliate links like Ebay, Amazon, and others. ... Dangers are exaggerated in my opinion because gas is so easy to smell and you have to have a death wish to let your boat leak gas and try and start it. SloopJonB Super Anarchist ...
The series of Pearsons that contained the Pearson 26, 30, 10M and 36 were some of Bill Shaw's best designs., The rig proportions were a product of their time. These boats were all designed for the 155% genoas of the era (and the earliest ones were designed for 180% genoas..Eeek!)
Pearson 10M is a 33′ 0″ / 10.1 m monohull sailboat designed by William Shaw and built by Pearson Yachts between 1973 and 1981. Great choice! Your favorites are temporarily saved for this session. Sign in to save them permanently, access them on any device, and receive relevant alerts. ... Sail area in square feet, derived by adding the ...
Hi Matt, I know you will enjoy your 10 meter. We live in the Miami area and had her out in 20-25 kt. winds on the Ocean and she handled very well. I was used to a 1976 Pearson 424 Cutter/Ketch so I was skeptical of the 10 Meter. We made several trips to Newport R I from Miami with the 424, 11 days on the outside straight thru. I want to do this with our 10 Meter next.
In this case, it's the new Pearson 10M. 33 feet of company talent coming together in a boat that has been designed and executed to fill a specific role, viz: to offer more boat for the money in 33 feet than anyone could imagine possible in today's inflationary economy at absolutely no sacrifice in Pearson quality standards. Step aboard.
new to this Pearson 33 10 meter boat .. can someone tell me, in say 25 knot wind and say 3 to 4 ft coastal seas, how close in degrees can this boat sail on the wind and how far in degrees can one heel till the sails start dumping wind.. looking for opinion based on experience in a Pearson 33 10 meter boat.
Pearson 36-2 Houston, TX / Rock Hall, MD. Feb 21, 2008. #18. Grace Products. Cappep,u000bu000bWhat I ment by Grace products was that the glass mat (laminants), epoxy rein, hardners, curing agents, etc. were produced by W.R. Grace Corp. They were prety much the leader of fiberglass products at the time.
The 10M also has more sail area. The 10M will point higher than the 33-2. Both boats are performance oriented (if you have the fin keeled 33-2), but the 10M has more sail area and a longer waterline, thus yeilding a lower PHRF rating. I recommend to keep looking for the 10M. Some of the ones on the east coast have some issues.
Re: Pearson 10M. Hello, I owned 1969 Pearson 35-- 1979 Pearson 424 Cutter / Ketch and presently have my 1975 Pearson 10 Meter. I like the older Pearsons as they are built very heavy and sail very well in heavy seas. We went to Newport R I. a few times on the outside. Took us 11 days, day and night sailing.
1. Freeboard on the Commander is somewhat low (top), which sometimes makes for a wet, but exciting ride in bumpy conditions. 1. The generous cockpit featured on the Commander realistically seats six while under sail, but will accommodate more while at anchor with the tiller folded up. The cockpit is self-draining, but could use larger drains.
2.2 Physicochemical characterization and sampling scheme. Samples collected from Mine 2 TI were taken from two different points in the reservoir (see Supplementary Figure S2) which include a floating platform at the deepest point of the reservoir and the outflow dam.The outflow dam represents an approximate average of the overall water column and is ~2 m deep at the sampling location (samples ...
Boat: 1975 Pearson 10 Meter. Posts: 35 Re: Pearson 33 / 10 meter. I had Pearson`s for many years. I have a 1975 10 meter and very happy with her on Ocean sailing , easy to handle and very responsive. ... Product or Service Reviews & Evaluations: 0: 13-08-2008 02:58: SIGNET MK170.4B Depth Meter Wanted: Capt Roy: Classifieds Archive: 0: 13-06 ...
Boat Review Forum. SailNet is a forum community dedicated to Sailing enthusiasts. Come join the discussion about sailing, modifications, classifieds, ... I've been looking at the Pearson 31 and the Pearson 10-M, mostly because I think they deliver a lot of boat for the money.
It is estimated that sea levels will rise at least 1 foot (ft) (0.3 meters (m)) above year 2000 levels by the century's end (NOAA 2022, unpaginated). However, some research suggests the magnitude may be far greater than previously predicted due to recent rapid ice loss from Greenland and Antarctica (Rignot and Kanagaratnam 2006, pp. 989-990).
Pearson 10 meter. AWT2_Sail · Nov 6, 2021. Follow AWT2_Sail · Nov 6, 2021. SailNet is a forum community dedicated to Sailing enthusiasts. ... classifieds, troubleshooting, repairs, reviews, maintenance, and more!